Cell Metabolism ( IF 29.0 ) Pub Date : 2021-09-23 , DOI: 10.1016/j.cmet.2021.09.002 Samantha M Morrissey 1 , Fan Zhang 2 , Chuanlin Ding 3 , Diego Elias Montoya-Durango 3 , Xiaoling Hu 3 , Chenghui Yang 4 , Zhen Wang 5 , Fang Yuan 6 , Matthew Fox 7 , Huang-Ge Zhang 8 , Haixun Guo 9 , David Tieri 10 , Maiying Kong 11 , Corey T Watson 10 , Robert A Mitchell 3 , Xiang Zhang 6 , Kelly M McMasters 3 , Jian Huang 5 , Jun Yan 1
|
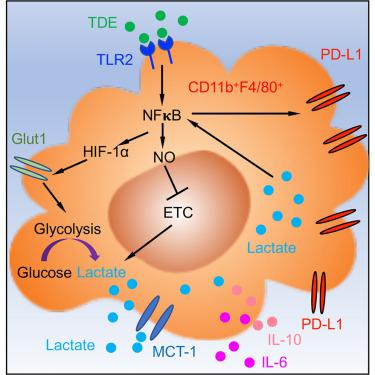
One of the defining characteristics of a pre-metastatic niche, a fundamental requirement for primary tumor metastasis, is infiltration of immunosuppressive macrophages. How these macrophages acquire their phenotype remains largely unexplored. Here, we demonstrate that tumor-derived exosomes (TDEs) polarize macrophages toward an immunosuppressive phenotype characterized by increased PD-L1 expression through NF-kB-dependent, glycolytic-dominant metabolic reprogramming. TDE signaling through TLR2 and NF-κB leads to increased glucose uptake. TDEs also stimulate elevated NOS2, which inhibits mitochondrial oxidative phosphorylation resulting in increased conversion of pyruvate to lactate. Lactate feeds back on NF-κB, further increasing PD-L1. Analysis of metastasis-negative lymph nodes of non-small-cell lung cancer patients revealed that macrophage PD-L1 positively correlates with levels of GLUT-1 and vesicle release gene YKT6 from primary tumors. Collectively, our study provides a novel mechanism by which macrophages within a pre-metastatic niche acquire their immunosuppressive phenotype and identifies an important link among exosomes, metabolism, and metastasis.
中文翻译:

肿瘤衍生的外泌体通过糖酵解优势代谢重编程驱动转移前生态位中的免疫抑制巨噬细胞
转移前生态位的定义特征之一是原发性肿瘤转移的基本要求,是免疫抑制性巨噬细胞的浸润。这些巨噬细胞如何获得它们的表型在很大程度上仍未被探索。在这里,我们证明肿瘤衍生的外泌体 (TDE) 将巨噬细胞极化为免疫抑制表型,其特征是通过 NF-kB 依赖性、糖酵解主导的代谢重编程增加 PD-L1 表达。通过 TLR2 和 NF-κB 的 TDE 信号传导导致葡萄糖摄取增加。TDE 还刺激 NOS2 升高,从而抑制线粒体氧化磷酸化,导致丙酮酸向乳酸的转化增加。乳酸反馈 NF-κB,进一步增加 PD-L1。对非小细胞肺癌患者转移阴性淋巴结的分析显示,巨噬细胞 PD-L1 与原发性肿瘤的 GLUT-1 和囊泡释放基因 YKT6 水平呈正相关。总的来说,我们的研究提供了一种新的机制,通过该机制,转移前生态位中的巨噬细胞获得其免疫抑制表型,并确定了外泌体、代谢和转移之间的重要联系。



























 京公网安备 11010802027423号
京公网安备 11010802027423号