当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nitrosobenzene-Enabled Chiral Phosphoric Acid Catalyzed Enantioselective Construction of Atropisomeric N-Arylbenzimidazoles
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2021-09-23 , DOI: 10.1002/anie.202111251 Qian-Jin An 1 , Wang Xia 1 , Wei-Yi Ding 1 , Huan-Huan Liu 1 , Shao-Hua Xiang 1 , Yong-Bin Wang 1 , Guofu Zhong 2 , Bin Tan 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2021-09-23 , DOI: 10.1002/anie.202111251 Qian-Jin An 1 , Wang Xia 1 , Wei-Yi Ding 1 , Huan-Huan Liu 1 , Shao-Hua Xiang 1 , Yong-Bin Wang 1 , Guofu Zhong 2 , Bin Tan 1
Affiliation
|
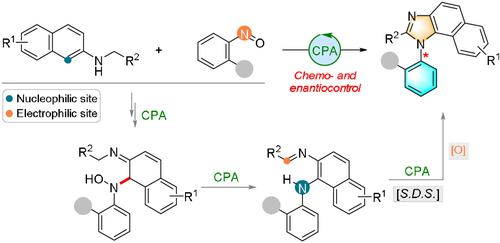
A benzimidazole ring formation strategy for the synthesis of axially chiral N-arylbenzimidazoles by means of chiral phosphoric acid catalysis is presented. Two sets of conditions were developed to transform two classes of 2-naphthylamine derivatives into structurally diverse N-arylbenzimidazole atropisomers with excellent chemo- and regioselectivity as well as high levels of enantiocontrol.

中文翻译:

亚硝基苯促进手性磷酸催化的阻转异构体 N-芳基苯并咪唑的对映选择性构建
提出了一种通过手性磷酸催化合成轴向手性N-芳基苯并咪唑的苯并咪唑成环策略。开发了两组条件以将两类 2-萘胺衍生物转化为结构多样的N-芳基苯并咪唑阻转异构体,具有优异的化学和区域选择性以及高水平的对映控制。

更新日期:2021-11-08

中文翻译:

亚硝基苯促进手性磷酸催化的阻转异构体 N-芳基苯并咪唑的对映选择性构建
提出了一种通过手性磷酸催化合成轴向手性N-芳基苯并咪唑的苯并咪唑成环策略。开发了两组条件以将两类 2-萘胺衍生物转化为结构多样的N-芳基苯并咪唑阻转异构体,具有优异的化学和区域选择性以及高水平的对映控制。












































 京公网安备 11010802027423号
京公网安备 11010802027423号