Applied Surface Science ( IF 6.3 ) Pub Date : 2021-09-23 , DOI: 10.1016/j.apsusc.2021.151374 Xu Zhang 1, 2 , Shaobin Yang 2 , Shuwei Tang 2 , Dongyang Hao 2 , Sinan Li 3
|
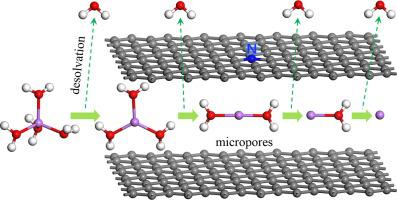
The size of the nanopores in which the solvated ions in water-based electrolytes of electric double-layer capacitors (EDLCs) can be desolvated and the reasons for desolvation should be further studied. In this study, we calculate the stable structures of Li++nH2O (n = 1, 2, 3, 4) and Na++nH2O in nitrogen-doped bilayer graphene with different interlayer spacing (BGN-x, x = 4, 5, 6, 7, 8 Å) using first-principles calculations to simulate the desolvation behavior for Li++nH2O and Na++nH2O in nanopores of nitrogen-doped carbon electrode materials. The calculation results show that the number of H2O bound to Li+ in the BGN-x is related to the size of the interlayer spacing. The increase or decrease in the number of H2O changes the solvated structure of Li+, and the change of OH-π interaction makes the solvated structure unstable, so desolvation will occur in micropores with different sizes. When the interlayer spacing decreases (about 5 Å), that is, the nanopore size becomes smaller, the equilibrium between the Li+ or H atom of H2O and the large π bond (Li+-π, OH-π) in graphene is broken, and the interaction of Li+-π is stronger than OH-π so that Li++nH2O gradually removes H2O and occurs desolvation. After desolvation, the maximum relative capacitance can reach more than twice that of the mesopores and macropores. The value of relative capacitance has a great relationship with the number of H2O bound to Li+ and the size of interlayer spacing. The energy decomposition analysis indicates that the interactions of Li+-π and OH-π play a key role in the stability of the BGN-x@Li++nH2O structures during the optimization process. For Na++nH2O, the reduced interlayer spacing (approximately 4.5 Å) makes it desolvate. Compared with the case without nitrogen doping, the size of desolvation for Na++nH2O decreases. The relative capacitance changes little after desolvation, which indicates that the change of the solvated structures of Na++nH2O has little effect on the capacitance. The results explain the relationship among desolvation of solvated ions, stability of the structures, and capacitance, which is helpful to accurately match the electrolyte and electrode materials, and provide a theoretical explanation for the increase of capacitance in the nanopores of EDLCs.
中文翻译:

深入了解氮掺杂纳米孔对水溶液中溶剂化 Li+ 和 Na+ 结构去溶剂化的影响:第一性原理研究
双电层电容器(EDLCs)的水基电解质中溶剂化离子可以去溶剂化的纳米孔的大小和去溶剂化的原因应进一步研究。在这项研究中,我们计算Li的稳定结构+ + Ñ ħ 2 O(Ñ = 1,2,3,4)和Na + + Ñ ħ 2 ö在具有不同层间距氮掺杂的双层石墨烯(BGN- X , x = 4, 5, 6, 7, 8 Å) 使用第一性原理计算来模拟 Li + + n H 2 O 和 Na + + n的去溶剂化行为H 2 O 在氮掺杂碳电极材料的纳米孔中。计算结果表明,BGN- x中与Li +结合的H 2 O的数量与层间距的大小有关。H 2 O数量的增加或减少会改变 Li +的溶剂化结构,而 OH-π 相互作用的变化使溶剂化结构不稳定,因此在不同大小的微孔中会发生去溶剂化。当层间距减小(约 5 Å),即纳米孔尺寸变小时,H 2 O的 Li +或 H 原子与大 π 键(Li +-π, OH-π) 在石墨烯中被破坏,Li + -π的相互作用强于 OH-π,使得 Li + + n H 2 O 逐渐去除 H 2 O 并发生去溶剂化。去溶剂化后,最大相对电容可以达到中孔和大孔的两倍以上。相对电容值与与Li +结合的H 2 O的数量和层间距的大小有很大关系。能量分解分析表明,Li + -π 和 OH-π的相互作用对BGN- x @Li + + n H 2的稳定性起着关键作用优化过程中的 O 结构。对于 Na + + n H 2 O,减小的层间距(大约 4.5 Å)使其去溶剂化。与未掺杂氮的情况相比,Na + + n H 2 O的去溶剂化尺寸减小。去溶剂化后相对电容变化不大,说明Na + + n H 2的溶剂化结构发生了变化O 对电容的影响很小。结果解释了溶剂化离子去溶剂化、结构稳定性和电容之间的关系,有助于电解质和电极材料的精确匹配,并为EDLCs纳米孔中电容的增加提供理论解释。











































 京公网安备 11010802027423号
京公网安备 11010802027423号