Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2021-09-22 , DOI: 10.1016/j.jhazmat.2021.127254 Xuan-Yuan Pei 1 , Hong-Yu Ren 1 , Guo-Shuai Liu 2 , Guang-Li Cao 1 , Guo-Jun Xie 1 , De-Feng Xing 1 , Nan-Qi Ren 1 , Bing-Feng Liu 1
|
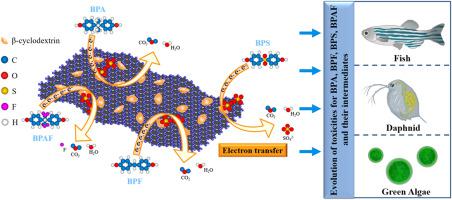
Bisphenols (BPs) are distributed in worldwide as typical environmental hormones, which potentially harm the ecological environment and human health. In this study, four BPs, i.e., bisphenol A, bisphenol F, bisphenol S, and bisphenol AF, were used as prototypes to identify the intrinsic differences in degradation mechanisms correlated with the molecular structures in peroxydisulfate (PDS)-based advanced oxidation processes (AOPs). Electron transfer was the main way of modified biochar to trigger the heterogenous catalysis of PDS, which can cause the degradation of BPs. Phenolic hydroxyl groups on bisphenol pollutants were considered as possible active sites, and the existence of substituents was the main reason for the differentiation in the degradation efficiency of various bisphenols. Results of ecotoxicity prediction showed that most intermediates produced by the degradation of BPs in the β-SB/PDS system, which was dominated by the electron transfer pathway, had a lower toxicity than the parent molecules, while the toxicity of several ring cleavage intermediates was higher. This study presents a simple modification scheme for the conversion of biochar into functional catalysts and provides insights into the mechanism of heterogeneous catalytic degradation mediated by modified biochar as well as the degradation differences of bisphenol pollutants and their potential ecotoxicity.
中文翻译:

β-环糊精功能化生物炭催化过硫酸盐降解双酚A及其类似物的非自由基机理及毒性分析
双酚类物质作为典型的环境激素分布于世界各地,对生态环境和人类健康具有潜在危害。在这项研究中,以双酚 A、双酚 F、双酚 S 和双酚 AF 四种 BP 作为原型,确定了与基于过二硫酸盐 (PDS) 的高级氧化过程中的分子结构相关的降解机制的内在差异。 AOP)。电子转移是改性生物炭触发PDS多相催化的主要方式,可引起BPs的降解。双酚类污染物上的酚羟基被认为是可能的活性位点,取代基的存在是导致各种双酚类物质降解效率存在差异的主要原因。生态毒性预测结果表明,在以电子传递途径为主的β-SB/PDS体系中,BPs降解产生的大多数中间体毒性低于母体分子,而几种环裂解中间体的毒性则为更高。本研究提出了一种将生物炭转化为功能催化剂的简单改性方案,并对改性生物炭介导的非均相催化降解机理以及双酚污染物的降解差异及其潜在的生态毒性提供了见解。










































 京公网安备 11010802027423号
京公网安备 11010802027423号