Chemosphere ( IF 8.1 ) Pub Date : 2021-09-21 , DOI: 10.1016/j.chemosphere.2021.132333 Sebastián Pérez 1 , Juan Muñoz-Saldaña 2 , Jesus Alberto Garcia-Nunez 3 , Nancy Acelas 1 , Elizabeth Flórez 1
|
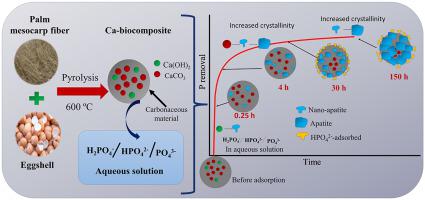
Phosphorus (P) adsorption from aqueous solutions is usually evaluated by monitoring the P concentration and employed kinetic models. In this work, three adsorbents obtained from eggshell (ES) and eggshell mixed with palm mesocarp fiber (ESF-1:1 and ESF-1:10) at different Ca(OH)2/CaCO3 compositions were evaluated, and the Ca–P species formed monitored as a function of time deconvoluting Fourier Transform Infrared (FTIR) spectra. At 0.25 h the ESF-1:10 (Ca(OH)2: 26.2 wt%) exhibited better adsorption performance of 35 mgg−1 while ESF-1:1 and ES (Ca(OH)2: 2.8 and 3.0 wt%) showed 26 and 4 mgg−1, respectively. Characteristic PO43− bands in apatite were corroborated by XRD and FTIR. It was found that the role of Ca(OH)2 in the adsorption ends before 0.25 h, and thereafter CaCO3 becomes the phase responsible for the removal of orthophosphate H2PO4−/HPO42−/PO43− ions. The results indicate a direct ligand exchange of CO32− for PO43− that takes place while increasing the apatite crystallinity. On the other hand, the P adsorption process is also dependent on P concentration. At low P concentrations, characteristic bands of PO43− in apatite were observed in FTIR, while at high concentrations, characteristic bands for adsorbed HPO42− were obtained. The obtained results give a relevant role to CaCO3 in P adsorption. Kinetic analysis for Ca-based biocomposites showed that the Avrami order kinetic model fits better for the adsorbents. For P adsorption isotherm process the Langmuir's isotherms showed a good fit, with a maximum adsorption capacity of 90.8, 134.0, and 67.9 mgg−1 for ES, ESF-1:1, and ESF-1:10, respectively.
中文翻译:

使用蛋壳-棕榈中果皮纤维的生物复合材料从水溶液中除磷过程中随着时间的推移解开 Ca-P 物种
从水溶液中吸附磷 (P) 通常通过监测 P 浓度和采用的动力学模型来评估。在这项工作中,评估了从蛋壳 (ES) 和蛋壳与棕榈中果皮纤维(ESF-1:1 和 ESF-1:10)在不同 Ca(OH) 2 /CaCO 3组成下获得的三种吸附剂,并评估了 Ca–作为时间解卷积傅立叶变换红外 (FTIR) 光谱的函数,监测形成的 P 物质。在 0.25 小时时,ESF-1:10 (Ca(OH) 2 : 26.2 wt%) 表现出更好的吸附性能,为 35 mg -1而 ESF-1:1 和 ES (Ca(OH) 2 : 2.8 和 3.0 wt%)分别显示 26 和 4 mg -1。特性 PO 4 3−磷灰石中的谱带通过 XRD 和 FTIR 得到证实。发现Ca(OH) 2在吸附中的作用在0.25小时前结束,此后CaCO 3成为负责去除正磷酸盐H 2 PO 4 - /HPO 4 2- /PO 4 3-离子的相。结果表明CO 3 2-与PO 4 3-的直接配体交换发生在增加磷灰石结晶度的同时。另一方面,P 吸附过程也依赖于 P 浓度。在低 P 浓度下,PO 4 3− 的特征带在 FTIR 中观察到磷灰石中的 , 而在高浓度下,获得了吸附 HPO 4 2- 的特征带。获得的结果给出了CaCO 3在P吸附中的相关作用。Ca 基生物复合材料的动力学分析表明,Avrami 有序动力学模型更适合吸附剂。对于 P 吸附等温线过程,Langmuir 等温线显示出良好的拟合,ES、ESF-1:1 和 ESF-1:10的最大吸附容量分别为 90.8、134.0 和 67.9 mg -1。









































 京公网安备 11010802027423号
京公网安备 11010802027423号