当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Structures and Spin States of Iron(II) Complexes of Isomeric 2,6-Di(1,2,3-triazolyl)pyridine Ligands
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2021-09-21 , DOI: 10.1021/acs.inorgchem.1c02404 Izar Capel Berdiell 1 , Daniel J Davies 1 , Jack Woodworth 1 , Rafal Kulmaczewski 1 , Oscar Cespedes 2 , Malcolm A Halcrow 1
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2021-09-21 , DOI: 10.1021/acs.inorgchem.1c02404 Izar Capel Berdiell 1 , Daniel J Davies 1 , Jack Woodworth 1 , Rafal Kulmaczewski 1 , Oscar Cespedes 2 , Malcolm A Halcrow 1
Affiliation
|
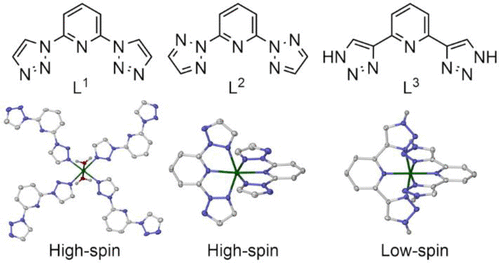
Iron(II) complex salts of 2,6-di(1,2,3-triazol-1-yl)pyridine (L1) are unexpectedly unstable in undried solvent. This is explained by the isolation of [Fe(L1)4(H2O)2][ClO4]2 and [Fe(NCS)2(L1)2(H2O)2]·L1, containing L1 bound as a monodentate ligand rather than in the expected tridentate fashion. These complexes associate into 44 grid structures through O–H···N hydrogen bonding; a solvate of a related 44 coordination framework, catena-[Cu(μ-L1)2(H2O)2][BF4]2, is also presented. The isomeric ligands 2,6-di(1,2,3-triazol-2-yl)pyridine (L2) and 2,6-di(1H-1,2,3-triazol-4-yl)pyridine (L3) bind to iron(II) in a more typical tridentate fashion. Solvates of [Fe(L3)2][ClO4]2 are low-spin and diamagnetic in the solid state and in solution, while [Fe(L2)2][ClO4]2 and [Co(L3)2][BF4]2 are fully high-spin. Treatment of L3 with methyl iodide affords 2,6-di(2-methyl-1,2,3-triazol-4-yl)pyridine (L4) and 2-(1-methyl-1,2,3-triazol-4-yl)-6-(2-methyl-1,2,3-triazol-4-yl)pyridine (L5). While salts of [Fe(L5)2]2+ are low-spin in the solid state, [Fe(L4)2][ClO4]2·H2O is high-spin, and [Fe(L4)2][ClO4]2·3MeNO2 exhibits a hysteretic spin transition to 50% completeness at T1/2 = 128 K (ΔT1/2 = 6 K). This transition proceeds via a symmetry-breaking phase transition to an unusual low-temperature phase containing three unique cation sites with high-spin, low-spin, and 1:1 mixed-spin populations. The unusual distribution of the spin states in the low-temperature phase reflects “spin-state frustration” of the mixed-spin cation site by an equal number of high-spin and low-spin nearest neighbors. Gas-phase density functional theory calculations reproduce the spin-state preferences of these and some related complexes. These highlight the interplay between the σ-basicity and π-acidity of the heterocyclic donors in this ligand type, which have opposing influences on the molecular ligand field. The Brønsted basicities of L1–L3 are very sensitive to the linkage isomerism of their triazolyl donors, which explains why their iron complex spin states show more variation than the better-known iron(II)/2,6-dipyrazolylpyridine system.
中文翻译:

异构体2,6-二(1,2,3-三唑基)吡啶配体的铁(II)配合物的结构和自旋态
2,6-二(1,2,3-三唑-1-基)吡啶(L 1 )的铁(II)络盐在未干燥溶剂中出乎意料地不稳定。这是通过[Fe(L 1 ) 4 (H 2 O) 2 ][ClO 4 ] 2和[Fe(NCS) 2 (L 1 ) 2 (H 2 O) 2 ]·L 1 的分离来解释的,其中含有L 1作为单齿配体而不是以预期的三齿方式结合。这些配合物通过O-H···N氢键结合成4 4网格结构;相关4 4协调框架的溶剂化物,还提供了catena -[Cu(μ-L 1 ) 2 (H 2 O) 2 ][BF 4 ] 2。异构配体 2,6-二(1,2,3-三唑-2-基)吡啶 (L 2 ) 和 2,6-二(1 H -1,2,3-三唑-4-基)吡啶 ( L 3 ) 以更典型的三齿方式与铁(II) 结合。[Fe(L 3 ) 2 ][ClO 4 ] 2 的溶剂化物在固态和溶液中是低自旋和抗磁性的,而 [Fe(L 2 ) 2 ][ClO 4 ] 2和 [Co(L 3 ) 2][BF 4 ] 2是完全高自旋的。用碘甲烷处理 L 3得到 2,6-二(2-甲基-1,2,3-三唑-4-基)吡啶 (L 4 ) 和 2-(1-甲基-1,2,3-三唑-4-基)-6-(2-甲基-1,2,3-三唑-4-基)吡啶(L 5 )。[Fe(L 5 ) 2 ] 2+ 的盐在固态下是低自旋的,[Fe(L 4 ) 2 ][ClO 4 ] 2 ·H 2 O 是高自旋的,[Fe(L 4 ) 2 ][ClO 4 ] 2 ·3MeNO 2在T 1/2 = 128 K (Δ T 1/2= 6 K)。这种转变通过破坏对称性的相变进行到一个不寻常的低温相,该相包含三个独特的阳离子位点,具有高自旋、低自旋和 1:1 混合自旋种群。低温相中自旋态的异常分布反映了混合自旋阳离子位点的“自旋态受挫”由相同数量的高自旋和低自旋最近邻位点引起。气相密度泛函理论计算再现了这些和一些相关配合物的自旋状态偏好。这些突出了这种配体类型中杂环供体的σ-碱性和π-酸性之间的相互作用,这对分子配体领域具有相反的影响。L 1 –L 3的 Brønsted 碱度 对它们的三唑基供体的连接异构现象非常敏感,这解释了为什么它们的铁络合物自旋状态比众所周知的铁(II)/2,6-二吡唑基吡啶系统显示出更多的变化。
更新日期:2021-10-04
中文翻译:

异构体2,6-二(1,2,3-三唑基)吡啶配体的铁(II)配合物的结构和自旋态
2,6-二(1,2,3-三唑-1-基)吡啶(L 1 )的铁(II)络盐在未干燥溶剂中出乎意料地不稳定。这是通过[Fe(L 1 ) 4 (H 2 O) 2 ][ClO 4 ] 2和[Fe(NCS) 2 (L 1 ) 2 (H 2 O) 2 ]·L 1 的分离来解释的,其中含有L 1作为单齿配体而不是以预期的三齿方式结合。这些配合物通过O-H···N氢键结合成4 4网格结构;相关4 4协调框架的溶剂化物,还提供了catena -[Cu(μ-L 1 ) 2 (H 2 O) 2 ][BF 4 ] 2。异构配体 2,6-二(1,2,3-三唑-2-基)吡啶 (L 2 ) 和 2,6-二(1 H -1,2,3-三唑-4-基)吡啶 ( L 3 ) 以更典型的三齿方式与铁(II) 结合。[Fe(L 3 ) 2 ][ClO 4 ] 2 的溶剂化物在固态和溶液中是低自旋和抗磁性的,而 [Fe(L 2 ) 2 ][ClO 4 ] 2和 [Co(L 3 ) 2][BF 4 ] 2是完全高自旋的。用碘甲烷处理 L 3得到 2,6-二(2-甲基-1,2,3-三唑-4-基)吡啶 (L 4 ) 和 2-(1-甲基-1,2,3-三唑-4-基)-6-(2-甲基-1,2,3-三唑-4-基)吡啶(L 5 )。[Fe(L 5 ) 2 ] 2+ 的盐在固态下是低自旋的,[Fe(L 4 ) 2 ][ClO 4 ] 2 ·H 2 O 是高自旋的,[Fe(L 4 ) 2 ][ClO 4 ] 2 ·3MeNO 2在T 1/2 = 128 K (Δ T 1/2= 6 K)。这种转变通过破坏对称性的相变进行到一个不寻常的低温相,该相包含三个独特的阳离子位点,具有高自旋、低自旋和 1:1 混合自旋种群。低温相中自旋态的异常分布反映了混合自旋阳离子位点的“自旋态受挫”由相同数量的高自旋和低自旋最近邻位点引起。气相密度泛函理论计算再现了这些和一些相关配合物的自旋状态偏好。这些突出了这种配体类型中杂环供体的σ-碱性和π-酸性之间的相互作用,这对分子配体领域具有相反的影响。L 1 –L 3的 Brønsted 碱度 对它们的三唑基供体的连接异构现象非常敏感,这解释了为什么它们的铁络合物自旋状态比众所周知的铁(II)/2,6-二吡唑基吡啶系统显示出更多的变化。



























 京公网安备 11010802027423号
京公网安备 11010802027423号