当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Arylfurans by Organic-Solvent-Free Method Using Phosphoric Acid as a Solvent and Catalyst
ChemistrySelect ( IF 1.9 ) Pub Date : 2021-09-22 , DOI: 10.1002/slct.202103038 Ibrahim Yusuf Ajibola 1 , Liankun Ai 1 , Baolin Li 1
ChemistrySelect ( IF 1.9 ) Pub Date : 2021-09-22 , DOI: 10.1002/slct.202103038 Ibrahim Yusuf Ajibola 1 , Liankun Ai 1 , Baolin Li 1
Affiliation
|
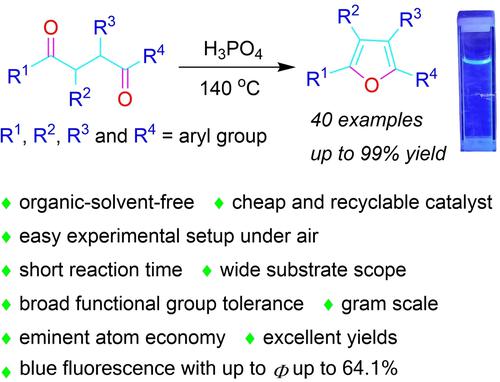
Phosphoric acid when being used as a solvent readily catalysed the cyclization reaction of 1,4-dicarbonyl compounds to furnish a broad range of arylfuran compounds using the Paal-Knorr furan synthetic method. The advantages of this synthetic method include an organic-solvent-free system, cheap and recyclable catalyst, easy experimental setup under air, short reaction time, wide substrate scope, broad functional group tolerance, eminent atom economy and excellent yields. The recovered acidic catalyst could be reused many times without significant loss in the catalytic activity. The photophysical properties of some selected arylfurans were examined in dilute solution. We found that most of the arylfurans emit in deep blue or violet region with moderate to high absolute quantum yields.
中文翻译:

以磷酸为溶剂和催化剂的无有机溶剂法合成芳基呋喃
磷酸用作溶剂时很容易催化 1,4-二羰基化合物的环化反应,使用 Paal-Knorr 呋喃合成方法提供范围广泛的芳基呋喃化合物。这种合成方法的优点包括无有机溶剂体系、廉价且可回收的催化剂、空气下实验设置简单、反应时间短、底物范围广、官能团耐受性广、原子经济性高、产率高。回收的酸性催化剂可以重复使用多次,催化活性没有明显损失。在稀溶液中检查了一些选定的芳基呋喃的光物理性质。我们发现大多数芳基呋喃在深蓝色或紫色区域发射,绝对量子产率中等至高。
更新日期:2021-09-22
中文翻译:

以磷酸为溶剂和催化剂的无有机溶剂法合成芳基呋喃
磷酸用作溶剂时很容易催化 1,4-二羰基化合物的环化反应,使用 Paal-Knorr 呋喃合成方法提供范围广泛的芳基呋喃化合物。这种合成方法的优点包括无有机溶剂体系、廉价且可回收的催化剂、空气下实验设置简单、反应时间短、底物范围广、官能团耐受性广、原子经济性高、产率高。回收的酸性催化剂可以重复使用多次,催化活性没有明显损失。在稀溶液中检查了一些选定的芳基呋喃的光物理性质。我们发现大多数芳基呋喃在深蓝色或紫色区域发射,绝对量子产率中等至高。











































 京公网安备 11010802027423号
京公网安备 11010802027423号