当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Micelle-Mediated Trimerization of Ynals to Orthogonally Substituted 4H-Pyrans in Water: Downstream Rearrangement to Bioactive 2,8-dioxabicyclo[3.3.1]nona-3,6-diene Frameworks
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2021-09-22 , DOI: 10.1002/ejoc.202101122 Showkat Rashid 1 , Bilal Bhat 2 , Goverdhan Mehta 3
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2021-09-22 , DOI: 10.1002/ejoc.202101122 Showkat Rashid 1 , Bilal Bhat 2 , Goverdhan Mehta 3
Affiliation
|
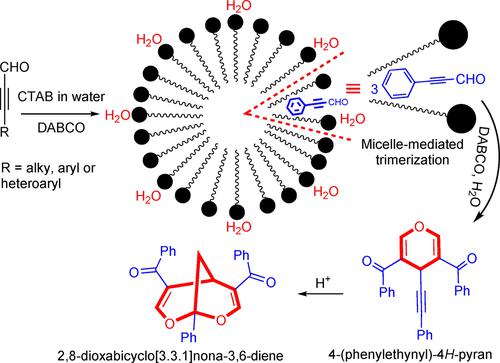
An efficient and environmentally benign protocol has been devised to induce trimerization of ynals to 4H-pyrans in water. This strategy has been successfully tested on a range of aliphatic, aromatic and hetero-aromatic substrates. Acid-mediated rearrangement of 4H-pyrans to interesting 2,8-dioxabicyclo[3.3.1]nona-3,6-dienes has been also demonstrated. The location of the reaction site in the micelle‘s palisade region was established through proton NMR studies.

中文翻译:

胶束介导的 Ynals 在水中三聚到正交取代的 4H-吡喃:下游重排为生物活性 2,8-二氧杂双环 [3.3.1]nona-3,6-二烯骨架
已设计出一种高效且环境友好的方案,以在水中将 ynals 三聚化为 4 H-吡喃。该策略已在一系列脂肪族、芳香族和杂芳香族底物上成功测试。还证明了酸介导的 4 H-吡喃重排为有趣的 2,8-二氧杂双环 [3.3.1] 壬 -3,6-二烯。通过质子核磁共振研究确定了胶束栅栏区域中反应位点的位置。

更新日期:2021-09-22

中文翻译:

胶束介导的 Ynals 在水中三聚到正交取代的 4H-吡喃:下游重排为生物活性 2,8-二氧杂双环 [3.3.1]nona-3,6-二烯骨架
已设计出一种高效且环境友好的方案,以在水中将 ynals 三聚化为 4 H-吡喃。该策略已在一系列脂肪族、芳香族和杂芳香族底物上成功测试。还证明了酸介导的 4 H-吡喃重排为有趣的 2,8-二氧杂双环 [3.3.1] 壬 -3,6-二烯。通过质子核磁共振研究确定了胶束栅栏区域中反应位点的位置。












































 京公网安备 11010802027423号
京公网安备 11010802027423号