当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Organocatalytic Asymmetric Arylation of p-Quinone Phosphonates: A Green Access to Biaryl Monophosphorus Ligands
Organic Letters ( IF 4.9 ) Pub Date : 2021-09-22 , DOI: 10.1021/acs.orglett.1c02852 Guodong Sun 1, 2 , Zhuofei Deng 3 , Zhonghua Luo 3 , Zhongqing Wang 3 , Jiancun Zhang 1
Organic Letters ( IF 4.9 ) Pub Date : 2021-09-22 , DOI: 10.1021/acs.orglett.1c02852 Guodong Sun 1, 2 , Zhuofei Deng 3 , Zhonghua Luo 3 , Zhongqing Wang 3 , Jiancun Zhang 1
Affiliation
|
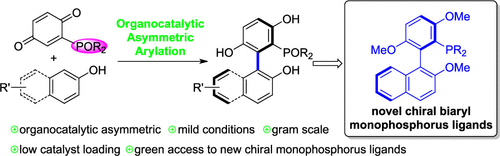
Herein, we report a highly efficient organocatalytic asymmetric synthesis of axially chiral biaryl phosphonates with p-quinone phosphonates and 2-naphthols via CPA-catalyzed asymmetric arylations. A series of chiral biaryl monophosphonates were obtained in excellent yields and enantioselectivities (up to 99% yield and 95% ee). This reaction could be operated at a gram scale with a low catalyst loading (0.5 mol %). Remarkably, our approach provides a green and ready access to chiral biaryl monophosphorus ligands. Compound 4ca was successfully converted to novel chiral biaryl monophosphorus ligands 7a, 7b, and 8 with high enantioselectivities in three steps.
中文翻译:

对醌膦酸酯的有机催化不对称芳基化:获得联芳基单磷配体的绿色途径
在此,我们报告了一种通过 CPA 催化的不对称芳基化高效有机催化不对称合成轴向手性联芳基膦酸酯与对醌膦酸酯和 2-萘酚的方法。以优异的产率和对映选择性(高达 99% 的产率和 95% ee)获得了一系列手性联芳基单膦酸酯。该反应可以在低催化剂负载量(0.5 mol%)下以克规模进行。值得注意的是,我们的方法提供了一种绿色且易于使用的手性联芳基单磷配体。化合物4ca通过三个步骤成功地转化为具有高对映选择性的新型手性联芳基单磷配体7a、7b和8。
更新日期:2021-10-01
中文翻译:

对醌膦酸酯的有机催化不对称芳基化:获得联芳基单磷配体的绿色途径
在此,我们报告了一种通过 CPA 催化的不对称芳基化高效有机催化不对称合成轴向手性联芳基膦酸酯与对醌膦酸酯和 2-萘酚的方法。以优异的产率和对映选择性(高达 99% 的产率和 95% ee)获得了一系列手性联芳基单膦酸酯。该反应可以在低催化剂负载量(0.5 mol%)下以克规模进行。值得注意的是,我们的方法提供了一种绿色且易于使用的手性联芳基单磷配体。化合物4ca通过三个步骤成功地转化为具有高对映选择性的新型手性联芳基单磷配体7a、7b和8。











































 京公网安备 11010802027423号
京公网安备 11010802027423号