Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Understanding liquid–liquid equilibria in binary mixtures of hydrocarbons with a thermally robust perarylphosphonium-based ionic liquid
RSC Advances ( IF 3.9 ) Pub Date : 2021-09-22 , DOI: 10.1039/d1ra06268a Santosh R P Bandlamudi 1 , Jimmie L McGehee 1 , Albaraa D Mando 1 , Mohammad Soltani 2 , C Heath Turner 3 , James H Davis 2 , Kevin N West 1 , Brooks D Rabideau 1
RSC Advances ( IF 3.9 ) Pub Date : 2021-09-22 , DOI: 10.1039/d1ra06268a Santosh R P Bandlamudi 1 , Jimmie L McGehee 1 , Albaraa D Mando 1 , Mohammad Soltani 2 , C Heath Turner 3 , James H Davis 2 , Kevin N West 1 , Brooks D Rabideau 1
Affiliation
|
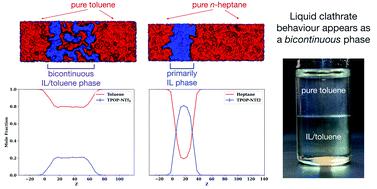
Binary mixtures of hydrocarbons and a thermally robust ionic liquid (IL) incorporating a perarylphosphonium-based cation are investigated experimentally and computationally. Experimentally, it is seen that excess toluene added to the IL forms two distinct liquid phases, an “ion-rich” phase of fixed composition and a phase that is nearly pure toluene. Conversely, n-heptane is observed to be essentially immiscible in the neat IL. Molecular dynamics simulations capture both of these behaviours. Furthermore, the simulated composition of the toluene-rich IL phase is within 10% of the experimentally determined composition. Additional simulations are performed on the binary mixtures of the IL and ten other small hydrocarbons having mixed aromatic/aliphatic character. It is found that hydrocarbons with a predominant aliphatic character are largely immiscible with the IL, while those with a predominant aromatic character readily mix with the IL. A detailed analysis of the structure and energetic changes that occur on mixing reveals the nature of the ion-rich phase. The simulations show a bicontinuous phase with hydrocarbon uptake akin to absorption and swelling by a porous absorbent. Aromatic hydrocarbons are driven into the neat IL via dispersion forces with the IL cations and, to a lesser extent, the IL anions. The ion–ion network expands to accommodate the hydrocarbons, yet maintains a core connective structure. At a certain loading, this network becomes stretched to its limit. The energetic penalty associated with breaking the core connective network outweighs the gain from new hydrocarbon–IL interactions, leaving additional hydrocarbons in the neat phase. The spatially alternating charge of the expanded IL network is shown to interact favourably with the stacked aromatic subphase, something not possible for aliphatic hydrocarbons.
中文翻译:

使用耐热全芳基鏻基离子液体了解烃类二元混合物中的液-液平衡
通过实验和计算研究了碳氢化合物和耐热离子液体 (IL) 的二元混合物,其中包含基于过芳基鏻的阳离子。实验表明,添加到离子液体中的过量甲苯会形成两个不同的液相,一个是固定组成的“富离子”相,另一个是几乎纯甲苯的相。反之,n据观察,庚烷在纯 IL 中基本上不混溶。分子动力学模拟捕获了这两种行为。此外,富含甲苯的离子液体相的模拟组成与实验确定的组成相差在 10% 以内。对 IL 和其他十种具有混合芳香族/脂肪族特征的小分子烃的二元混合物进行了额外的模拟。研究发现,具有主要脂肪族特征的碳氢化合物在很大程度上与IL不混溶,而具有主要芳香族特征的碳氢化合物则很容易与IL混合。对混合时发生的结构和能量变化的详细分析揭示了富离子相的性质。模拟显示了双连续相,碳氢化合物的吸收类似于多孔吸收剂的吸收和膨胀。通过与 IL 阳离子以及较小程度的 IL 阴离子的分散力。离子-离子网络扩展以容纳碳氢化合物,但仍保持核心连接结构。在一定的负载下,该网络会被拉伸到极限。与破坏核心连接网络相关的能量损失超过了新的碳氢化合物-离子液体相互作用所带来的能量损失,从而使额外的碳氢化合物处于纯态。扩展的 IL 网络的空间交替电荷被证明可以与堆叠的芳香族亚相有利地相互作用,这对于脂肪族烃来说是不可能的。
更新日期:2021-09-22
中文翻译:

使用耐热全芳基鏻基离子液体了解烃类二元混合物中的液-液平衡
通过实验和计算研究了碳氢化合物和耐热离子液体 (IL) 的二元混合物,其中包含基于过芳基鏻的阳离子。实验表明,添加到离子液体中的过量甲苯会形成两个不同的液相,一个是固定组成的“富离子”相,另一个是几乎纯甲苯的相。反之,n据观察,庚烷在纯 IL 中基本上不混溶。分子动力学模拟捕获了这两种行为。此外,富含甲苯的离子液体相的模拟组成与实验确定的组成相差在 10% 以内。对 IL 和其他十种具有混合芳香族/脂肪族特征的小分子烃的二元混合物进行了额外的模拟。研究发现,具有主要脂肪族特征的碳氢化合物在很大程度上与IL不混溶,而具有主要芳香族特征的碳氢化合物则很容易与IL混合。对混合时发生的结构和能量变化的详细分析揭示了富离子相的性质。模拟显示了双连续相,碳氢化合物的吸收类似于多孔吸收剂的吸收和膨胀。通过与 IL 阳离子以及较小程度的 IL 阴离子的分散力。离子-离子网络扩展以容纳碳氢化合物,但仍保持核心连接结构。在一定的负载下,该网络会被拉伸到极限。与破坏核心连接网络相关的能量损失超过了新的碳氢化合物-离子液体相互作用所带来的能量损失,从而使额外的碳氢化合物处于纯态。扩展的 IL 网络的空间交替电荷被证明可以与堆叠的芳香族亚相有利地相互作用,这对于脂肪族烃来说是不可能的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号