Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis, cytotoxicity evaluation and molecular docking studies on 2′,4′-dihydroxy-6′-methoxy-3′,5′-dimethylchalcone derivatives
RSC Advances ( IF 3.9 ) Pub Date : 2021-09-22 , DOI: 10.1039/d1ra05445g Nopawit Khamto 1, 2 , Lada Chaichuang 1, 2 , Puracheth Rithchumpon 1, 2 , Worrapong Phupong 3 , Phuangthip Bhoopong 4 , Suriya Tateing 5 , Wilart Pompimon 6 , Natthawat Semakul 1, 7 , Ni-Orn Chomsri 8 , Puttinan Meepowpan 1, 7
RSC Advances ( IF 3.9 ) Pub Date : 2021-09-22 , DOI: 10.1039/d1ra05445g Nopawit Khamto 1, 2 , Lada Chaichuang 1, 2 , Puracheth Rithchumpon 1, 2 , Worrapong Phupong 3 , Phuangthip Bhoopong 4 , Suriya Tateing 5 , Wilart Pompimon 6 , Natthawat Semakul 1, 7 , Ni-Orn Chomsri 8 , Puttinan Meepowpan 1, 7
Affiliation
|
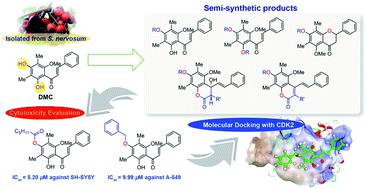
2′,4′-Dihydroxy-6′-methoxy-3′,5′-dimethylchalcone (DMC, 1) was isolated from seeds of Syzygium nervosum A.Cunn. ex DC. exhibiting intriguing biological activities. Herein, thirty three DMC derivatives including 4′-O-monosubstituted-DMC (2), 7-O-acylated-4-hydroxycoumarin derivatives (3), stilbene–coumarin derivatives (4), 2′,4′-disubstituted-DMC (5), and flavanone derivatives (6), were synthesised through acylation, alkylations, and sulfonylation. These semi-synthetic DMC derivatives were evaluated for in vitro cytotoxicity against six carcinoma cell lines. It was found that most derivatives exhibited higher cytotoxicity than DMC. In particular, 4′-O-caproylated-DMC (2b) and 4′-O-methylated-DMC (2g) displayed the strongest cytotoxicity against SH-SY5Y with IC50 values of 5.20 and 7.52 μM, respectively. Additionally, 4′-O-benzylated-DMC (2h) demonstrated the strongest cytotoxicity against A-549 and FaDu with IC50 values of 9.99 and 13.98 μM, respectively. Our structure–activity relationship (SAR) highlights the importance of 2′-OH and the derivatisation pattern of 4′-OH. Furthermore, molecular docking simulation studies shed further light on how these bioactive compounds interact with cyclin-dependent kinase 2 (CDK2).
中文翻译:

2',4'-二羟基-6'-甲氧基-3',5'-二甲基查尔酮衍生物的合成、细胞毒性评价及分子对接研究
2',4'-Dihydroxy-6'-methoxy-3',5'-dimethylchalcone (DMC, 1 ) 从Syzygium nervosum A.Cunn的种子中分离得到。前华盛顿特区。表现出有趣的生物活性。在此,33种DMC衍生物包括4'- O-单取代-DMC ( 2 )、7 - O-酰化-4-羟基香豆素衍生物( 3 )、芪-香豆素衍生物( 4 )、2',4'-二取代-DMC ( 5 )和黄烷酮衍生物( 6 )通过酰化、烷基化和磺酰化合成。这些半合成 DMC 衍生物在体外进行了评估对六种癌细胞系的细胞毒性。发现大多数衍生物表现出比DMC更高的细胞毒性。特别是, 4'- O-己酰化-DMC ( 2b ) 和4'- O-甲基化-DMC ( 2g ) 对SH-SY5Y 表现出最强的细胞毒性,IC 50值分别为5.20 和7.52 μM。此外,4'- O-苄基化-DMC ( 2h ) 对 A-549 和 FaDu 具有最强的细胞毒性,IC 50值分别为 9.99 和 13.98 μM。我们的构效关系 (SAR) 突出了 2'-OH 的重要性和 4'-OH 的衍生模式。此外,分子对接模拟研究进一步阐明了这些生物活性化合物如何与细胞周期蛋白依赖性激酶 2 (CDK2) 相互作用。
更新日期:2021-09-22
中文翻译:

2',4'-二羟基-6'-甲氧基-3',5'-二甲基查尔酮衍生物的合成、细胞毒性评价及分子对接研究
2',4'-Dihydroxy-6'-methoxy-3',5'-dimethylchalcone (DMC, 1 ) 从Syzygium nervosum A.Cunn的种子中分离得到。前华盛顿特区。表现出有趣的生物活性。在此,33种DMC衍生物包括4'- O-单取代-DMC ( 2 )、7 - O-酰化-4-羟基香豆素衍生物( 3 )、芪-香豆素衍生物( 4 )、2',4'-二取代-DMC ( 5 )和黄烷酮衍生物( 6 )通过酰化、烷基化和磺酰化合成。这些半合成 DMC 衍生物在体外进行了评估对六种癌细胞系的细胞毒性。发现大多数衍生物表现出比DMC更高的细胞毒性。特别是, 4'- O-己酰化-DMC ( 2b ) 和4'- O-甲基化-DMC ( 2g ) 对SH-SY5Y 表现出最强的细胞毒性,IC 50值分别为5.20 和7.52 μM。此外,4'- O-苄基化-DMC ( 2h ) 对 A-549 和 FaDu 具有最强的细胞毒性,IC 50值分别为 9.99 和 13.98 μM。我们的构效关系 (SAR) 突出了 2'-OH 的重要性和 4'-OH 的衍生模式。此外,分子对接模拟研究进一步阐明了这些生物活性化合物如何与细胞周期蛋白依赖性激酶 2 (CDK2) 相互作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号