当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Screening of transition metal doped copper clusters for CO2 activation
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2021-09-22 , DOI: 10.1039/d1cp02220b Máté Szalay 1 , Dániel Buzsáki 1 , Júlia Barabás 1 , Endre Faragó 1 , Ewald Janssens 2 , László Nyulászi 1, 3 , Tibor Höltzl 1, 3, 4
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2021-09-22 , DOI: 10.1039/d1cp02220b Máté Szalay 1 , Dániel Buzsáki 1 , Júlia Barabás 1 , Endre Faragó 1 , Ewald Janssens 2 , László Nyulászi 1, 3 , Tibor Höltzl 1, 3, 4
Affiliation
|
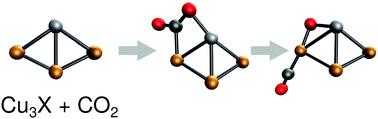
Activation of CO2 is the first step towards its reduction to more useful chemicals. Here we systematically investigate the CO2 activation mechanism on Cu3X (X is a first-row transition metal atom) using density functional theory computations. The CO2 adsorption energies and the activation mechanisms depend strongly on the selected dopant. The dopant electronegativity, the HOMO–LUMO gap and the overlap of the frontier molecular orbitals control the CO2 dissociation efficiency. Our calculations reveal that early transition metal-doped (Sc, Ti, V) clusters exhibit a high CO2 adsorption energy, a low activation barrier for its dissociation, and a facile regeneration of the clusters. Thus, early transition metal-doped copper clusters, particularly Cu3Sc, may be efficient catalysts for the carbon capture and utilization process.
中文翻译:

筛选用于 CO2 活化的过渡金属掺杂铜簇
CO 2 的活化是将其还原为更有用的化学品的第一步。在这里,我们使用密度泛函理论计算系统地研究了Cu 3 X(X 是第一行过渡金属原子)上的 CO 2活化机制。CO 2吸附能和活化机制在很大程度上取决于所选的掺杂剂。掺杂剂电负性、HOMO-LUMO 间隙和前沿分子轨道的重叠控制着 CO 2 的解离效率。我们的计算表明,早期掺杂过渡金属的 (Sc, Ti, V) 簇表现出高 CO 2吸附能,其解离的低活化势垒,以及簇的容易再生。因此,早期的过渡金属掺杂的铜簇,特别是Cu 3 Sc,可能是碳捕获和利用过程的有效催化剂。
更新日期:2021-09-22
中文翻译:

筛选用于 CO2 活化的过渡金属掺杂铜簇
CO 2 的活化是将其还原为更有用的化学品的第一步。在这里,我们使用密度泛函理论计算系统地研究了Cu 3 X(X 是第一行过渡金属原子)上的 CO 2活化机制。CO 2吸附能和活化机制在很大程度上取决于所选的掺杂剂。掺杂剂电负性、HOMO-LUMO 间隙和前沿分子轨道的重叠控制着 CO 2 的解离效率。我们的计算表明,早期掺杂过渡金属的 (Sc, Ti, V) 簇表现出高 CO 2吸附能,其解离的低活化势垒,以及簇的容易再生。因此,早期的过渡金属掺杂的铜簇,特别是Cu 3 Sc,可能是碳捕获和利用过程的有效催化剂。









































 京公网安备 11010802027423号
京公网安备 11010802027423号