当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural and electronic analysis of the octarepeat region of prion protein with four Cu2+ by polarizable MD and QM/MM simulations
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2021-09-13 , DOI: 10.1039/d1cp03187b Jorge Nochebuena 1 , Liliana Quintanar 2 , Alberto Vela 2 , G Andrés Cisneros 1
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2021-09-13 , DOI: 10.1039/d1cp03187b Jorge Nochebuena 1 , Liliana Quintanar 2 , Alberto Vela 2 , G Andrés Cisneros 1
Affiliation
|
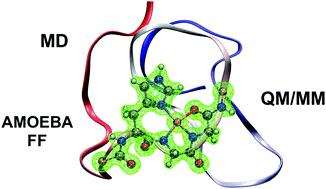
Prions have been linked to neurodegenerative diseases that affect various species of mammals including humans. The prion protein, located mainly in neurons, is believed to play the role of metal ion transporter. High levels of copper ions have been related to structural changes. A 32-residue region of the N-terminal domain, known as octarepeat, can bind up to four copper ions. Different coordination modes have been observed and are strongly dependent on Cu2+ concentration. Many theoretical studies carried out so far have focused on studying the coordination modes of a single copper ion. In this work we investigate the octarepeat region coordinated with four copper ions. Molecular dynamics (MD) and hybrid quantum mechanics/molecular mechanics (QM/MM) simulations using the polarizable AMOEBA force field have been carried out. The polarizable MD simulations starting from a fully extended conformation indicate that the tetra-Cu2+/octarepeat complex forms a globular structure. The globular form is stabilized by interactions between Cu2+ and tryptophan residues resulting in some coordination sites observed to be in close proximity, in agreement with experimental results. Subsequent QM/MM simulations on several snapshots suggests the system is in a high-spin quintet state, with all Cu2+ bearing one single electron, and all unpaired electrons are ferromagnetically coupled. NMR simulations on selected structures provides insights on the chemical shifts of the first shell ligands around the metals with respect to inter-metal distances.
中文翻译:

通过极化 MD 和 QM/MM 模拟对具有四个 Cu2+ 的朊病毒蛋白八重复区域进行结构和电子分析
朊病毒与影响包括人类在内的各种哺乳动物的神经退行性疾病有关。朊病毒蛋白主要位于神经元中,被认为发挥金属离子转运蛋白的作用。高含量的铜离子与结构变化有关。N 端结构域的 32 个残基区域(称为八角重复)最多可以结合四个铜离子。已经观察到不同的配位模式并且强烈依赖于Cu 2+浓度。迄今为止进行的许多理论研究都集中在研究单个铜离子的配位模式。在这项工作中,我们研究了与四个铜离子配位的八重区域。使用可极化 AMOEBA 力场进行了分子动力学 (MD) 和混合量子力学/分子力学 (QM/MM) 模拟。从完全伸展的构象开始的可极化MD模拟表明四-Cu 2+ /八重复复合物形成球状结构。球状形式通过 Cu 2+和色氨酸残基之间的相互作用而稳定,导致观察到一些配位位点非常接近,这与实验结果一致。随后对几个快照的 QM/MM 模拟表明该系统处于高自旋五重态,所有 Cu 2+都带有一个电子,并且所有不成对的电子都是铁磁耦合的。对选定结构的核磁共振模拟提供了有关金属周围第一壳配体相对于金属间距离的化学位移的见解。
更新日期:2021-09-22
中文翻译:

通过极化 MD 和 QM/MM 模拟对具有四个 Cu2+ 的朊病毒蛋白八重复区域进行结构和电子分析
朊病毒与影响包括人类在内的各种哺乳动物的神经退行性疾病有关。朊病毒蛋白主要位于神经元中,被认为发挥金属离子转运蛋白的作用。高含量的铜离子与结构变化有关。N 端结构域的 32 个残基区域(称为八角重复)最多可以结合四个铜离子。已经观察到不同的配位模式并且强烈依赖于Cu 2+浓度。迄今为止进行的许多理论研究都集中在研究单个铜离子的配位模式。在这项工作中,我们研究了与四个铜离子配位的八重区域。使用可极化 AMOEBA 力场进行了分子动力学 (MD) 和混合量子力学/分子力学 (QM/MM) 模拟。从完全伸展的构象开始的可极化MD模拟表明四-Cu 2+ /八重复复合物形成球状结构。球状形式通过 Cu 2+和色氨酸残基之间的相互作用而稳定,导致观察到一些配位位点非常接近,这与实验结果一致。随后对几个快照的 QM/MM 模拟表明该系统处于高自旋五重态,所有 Cu 2+都带有一个电子,并且所有不成对的电子都是铁磁耦合的。对选定结构的核磁共振模拟提供了有关金属周围第一壳配体相对于金属间距离的化学位移的见解。









































 京公网安备 11010802027423号
京公网安备 11010802027423号