当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Plasma Catalysis for Ammonia Synthesis: A Microkinetic Modeling Study on the Contributions of Eley–Rideal Reactions
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2021-09-21 , DOI: 10.1021/acssuschemeng.1c02713 Yannick Engelmann 1 , Kevin van ’t Veer 1, 2 , Yury Gorbanev 1 , Erik Cornelis Neyts 1 , William F. Schneider 3 , Annemie Bogaerts 1
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2021-09-21 , DOI: 10.1021/acssuschemeng.1c02713 Yannick Engelmann 1 , Kevin van ’t Veer 1, 2 , Yury Gorbanev 1 , Erik Cornelis Neyts 1 , William F. Schneider 3 , Annemie Bogaerts 1
Affiliation
|
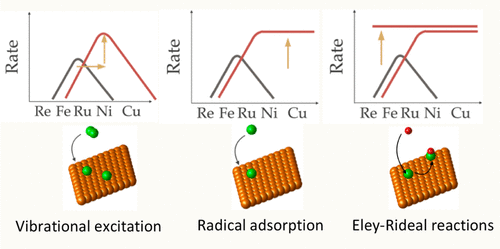
Plasma catalysis is an emerging new technology for the electrification and downscaling of NH3 synthesis. Increasing attention is being paid to the optimization of plasma catalysis with respect to the plasma conditions, the catalyst material, and their mutual interaction. In this work we use microkinetic models to study how the total conversion process is impacted by the combination of different plasma conditions and transition metal catalysts. We study how plasma-generated radicals and vibrationally excited N2 (present in a dielectric barrier discharge plasma) interact with the catalyst and impact the NH3 turnover frequencies (TOFs). Both filamentary and uniform plasmas are studied, based on plasma chemistry models that provided plasma phase speciation and vibrational distribution functions. The Langmuir–Hinshelwood reaction rate coefficients (i.e., adsorption reactions and subsequent reactions among adsorbates) are determined using conventional scaling relations. An additional set of Eley–Rideal reactions (i.e., direct reactions of plasma radicals with adsorbates) was added and a sensitivity analysis on the assumed reaction rate coefficients was performed. We first show the impact of different vibrational distribution functions on the catalytic dissociation of N2 and subsequent production of NH3, and we gradually include more radical reactions, to illustrate the contribution of these species and their corresponding reaction pathways. Analysis over a large range of catalysts indicates that different transition metals (metals such as Rh, Ni, Pt, and Pd) optimize the NH3TOFs depending on the population of the vibrational levels of N2. At higher concentrations of plasma-generated radicals, the NH3 TOFs become less dependent on the catalyst material, due to radical adsorptions on the more noble catalysts and Eley–Rideal reactions on the less noble catalysts.
中文翻译:

用于合成氨的等离子体催化:对 Eley-Rideal 反应贡献的微动力学建模研究
等离子体催化是一种新兴的新技术,用于 NH 3合成的电气化和降尺度。等离子体催化在等离子体条件、催化剂材料及其相互作用方面的优化受到越来越多的关注。在这项工作中,我们使用微动力学模型来研究不同等离子体条件和过渡金属催化剂的组合如何影响总转化过程。我们研究等离子体产生的自由基和振动激发的 N 2(存在于介质阻挡放电等离子体中)如何与催化剂相互作用并影响 NH 3周转频率 (TOF)。基于提供等离子体相形态和振动分布函数的等离子体化学模型,研究了丝状等离子体和均匀等离子体。Langmuir-Hinshelwood 反应速率系数(即吸附反应和吸附物之间的后续反应)是使用传统的比例关系确定的。添加了一组额外的 Eley-Rideal 反应(即等离子体自由基与吸附物的直接反应),并对假定的反应速率系数进行了敏感性分析。我们首先展示了不同振动分布函数对 N 2催化解离和随后产生 NH 3 的影响,我们逐渐包括更多的自由基反应,以说明这些物种的贡献及其相应的反应途径。对大量催化剂的分析表明,不同的过渡金属(如 Rh、Ni、Pt 和 Pd 等金属)根据 N 2振动能级的数量优化了 NH 3 TOF 。在较高浓度的等离子体产生的自由基下,NH 3 TOF 对催化剂材料的依赖性降低,这是由于自由基吸附在更贵的催化剂上,而 Eley-Rideal 反应在不太贵的催化剂上。
更新日期:2021-10-04
中文翻译:

用于合成氨的等离子体催化:对 Eley-Rideal 反应贡献的微动力学建模研究
等离子体催化是一种新兴的新技术,用于 NH 3合成的电气化和降尺度。等离子体催化在等离子体条件、催化剂材料及其相互作用方面的优化受到越来越多的关注。在这项工作中,我们使用微动力学模型来研究不同等离子体条件和过渡金属催化剂的组合如何影响总转化过程。我们研究等离子体产生的自由基和振动激发的 N 2(存在于介质阻挡放电等离子体中)如何与催化剂相互作用并影响 NH 3周转频率 (TOF)。基于提供等离子体相形态和振动分布函数的等离子体化学模型,研究了丝状等离子体和均匀等离子体。Langmuir-Hinshelwood 反应速率系数(即吸附反应和吸附物之间的后续反应)是使用传统的比例关系确定的。添加了一组额外的 Eley-Rideal 反应(即等离子体自由基与吸附物的直接反应),并对假定的反应速率系数进行了敏感性分析。我们首先展示了不同振动分布函数对 N 2催化解离和随后产生 NH 3 的影响,我们逐渐包括更多的自由基反应,以说明这些物种的贡献及其相应的反应途径。对大量催化剂的分析表明,不同的过渡金属(如 Rh、Ni、Pt 和 Pd 等金属)根据 N 2振动能级的数量优化了 NH 3 TOF 。在较高浓度的等离子体产生的自由基下,NH 3 TOF 对催化剂材料的依赖性降低,这是由于自由基吸附在更贵的催化剂上,而 Eley-Rideal 反应在不太贵的催化剂上。











































 京公网安备 11010802027423号
京公网安备 11010802027423号