Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
One-Pot Synthesis of Some New s-Triazole Derivatives and Their Potential Application for Water Decontamination
ACS Omega ( IF 3.7 ) Pub Date : 2021-09-22 , DOI: 10.1021/acsomega.1c03675 Zeinab A. Hozien, Ahmed F. M. EL-Mahdy, Laila S. A. Ali, Ahmad Abo Markeb, Hassan A. H. El-Sherief
ACS Omega ( IF 3.7 ) Pub Date : 2021-09-22 , DOI: 10.1021/acsomega.1c03675 Zeinab A. Hozien, Ahmed F. M. EL-Mahdy, Laila S. A. Ali, Ahmad Abo Markeb, Hassan A. H. El-Sherief
|
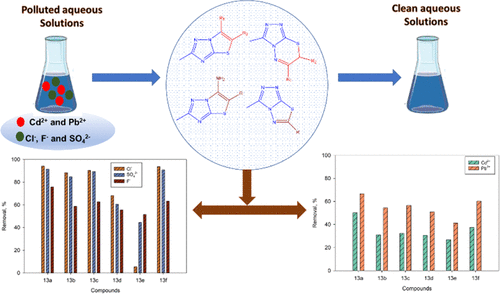
A rapid, efficient, and one-pot protocol has been developed for the synthesis of cyclized 2,6-dimethyl-5-substituted-thiazolo[3,2-b]-s-triazoles (3a–c) through the interaction of 5-methyl-1H-s-triazole-3-thiol (1) with aliphatic ketones (2a–d) in refluxing acetic acid in the presence of a catalytic amount of sulfuric acid (AcOH/H+) while with aromatic ketones (5a–d), a mixture of uncyclized 3-methyl-s-triazolylthioacetophenone derivatives (6a–d) and cyclized 6-aryl-2-methyl-thiazolo[3,2-b]-s-triazoles (7a–d) has been produced. With this catalytic system, inexpensive sulfuric acid was utilized as a catalyst, which prevented the production of poisonous and irritating halo carbonyl compounds. On the other hand, the interaction of s-triazole 1 with cyano compounds (9a,b) afforded the corresponding 6-amino-2-methyl-5-substituted-thiazolo[3,2-b]-s-triazoles (10a,b). Similarly, treatment of 4-amino-3-methyl-s-triazole-5-thiol (12) with aliphatic and aromatic ketones (2c and 5a–e) afforded directly 3-methyl-7H-s-triazolo[3,4-b]-1,3,4-thiadiazines (13a and 14a–e). Further, reaction of 12 with cyano compounds (9a,b) under the same reaction conditions yielded the corresponding 3-methyl-s-triazolo[3,4-b]-1,3,4-thiadiazole derivatives (15a,b). The reaction mechanism was studied, and the structures of all novel compounds were verified using spectroscopy and elemental analysis. Moreover, the potential application of the synthesized compounds toward heavy metal ions and inorganic anion removal from aqueous solution has been investigated. The removal effectiveness for metal ions reached up to 76.29%, while for inorganic anions it reached up to 100%, indicating that such synthesized compounds are promising adsorbents for water remediation.
中文翻译:

一些新型均三唑衍生物的一锅法合成及其在水净化中的潜在应用
开发了一种快速、高效、一锅法的方案,用于通过 5 的相互作用合成环化 2,6-二甲基-5-取代-噻唑并[3,2- b ]- s -三唑 ( 3a–c ) -甲基-1 H - s -三唑-3-硫醇 ( 1 ) 与脂肪酮 ( 2a–d ) 在催化量硫酸 (AcOH/H + ) 存在下在回流乙酸中反应,同时与芳香酮 ( 5a –d ),未环化的 3-甲基-s-三唑基硫代苯乙酮衍生物 ( 6a–d ) 和环化的 6-芳基-2-甲基-噻唑并[3,2- b ] -s-三唑 ( 7a–d ) 的混合物已被产生的。通过该催化系统,使用廉价的硫酸作为催化剂,防止了有毒且刺激性的卤代羰基化合物的产生。另一方面, s-三唑1与氰基化合物( 9a,b )相互作用得到相应的6-氨基-2-甲基-5-取代-噻唑并[3,2- b ] -s-三唑( 10a, b ).类似地,用脂肪族和芳香族酮( 2c和5a–e )处理4-氨基-3-甲基-s-三唑-5-硫醇( 12 )直接得到3-甲基-7H - s-三唑[3,4 - b ]-1,3,4-噻二嗪( 13a和14a–e )。此外, 12与氰基化合物( 9a,b )在相同反应条件下反应,得到相应的3-甲基-s-三唑并[3,4- b ]-1,3,4-噻二唑衍生物( 15a,b )。 研究了反应机理,并利用光谱和元素分析验证了所有新型化合物的结构。此外,还研究了合成的化合物在去除水溶液中的重金属离子和无机阴离子方面的潜在应用。对金属离子的去除率高达76.29%,对无机阴离子的去除率高达100%,表明此类合成的化合物是有前途的水体修复吸附剂。
更新日期:2021-10-06
中文翻译:

一些新型均三唑衍生物的一锅法合成及其在水净化中的潜在应用
开发了一种快速、高效、一锅法的方案,用于通过 5 的相互作用合成环化 2,6-二甲基-5-取代-噻唑并[3,2- b ]- s -三唑 ( 3a–c ) -甲基-1 H - s -三唑-3-硫醇 ( 1 ) 与脂肪酮 ( 2a–d ) 在催化量硫酸 (AcOH/H + ) 存在下在回流乙酸中反应,同时与芳香酮 ( 5a –d ),未环化的 3-甲基-s-三唑基硫代苯乙酮衍生物 ( 6a–d ) 和环化的 6-芳基-2-甲基-噻唑并[3,2- b ] -s-三唑 ( 7a–d ) 的混合物已被产生的。通过该催化系统,使用廉价的硫酸作为催化剂,防止了有毒且刺激性的卤代羰基化合物的产生。另一方面, s-三唑1与氰基化合物( 9a,b )相互作用得到相应的6-氨基-2-甲基-5-取代-噻唑并[3,2- b ] -s-三唑( 10a, b ).类似地,用脂肪族和芳香族酮( 2c和5a–e )处理4-氨基-3-甲基-s-三唑-5-硫醇( 12 )直接得到3-甲基-7H - s-三唑[3,4 - b ]-1,3,4-噻二嗪( 13a和14a–e )。此外, 12与氰基化合物( 9a,b )在相同反应条件下反应,得到相应的3-甲基-s-三唑并[3,4- b ]-1,3,4-噻二唑衍生物( 15a,b )。 研究了反应机理,并利用光谱和元素分析验证了所有新型化合物的结构。此外,还研究了合成的化合物在去除水溶液中的重金属离子和无机阴离子方面的潜在应用。对金属离子的去除率高达76.29%,对无机阴离子的去除率高达100%,表明此类合成的化合物是有前途的水体修复吸附剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号