Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Two-Dimensional Infrared Correlation Spectroscopy, Conductor-like Screening Model for Real Solvents, and Density Functional Theory Study on the Adsorption Mechanism of Polyvinylpolypyrrolidone for Effective Phenol Removal in an Aqueous Medium
ACS Omega ( IF 3.7 ) Pub Date : 2021-09-21 , DOI: 10.1021/acsomega.1c02699 Muhammad Ammar Mohammad Alwi 1 , Erna Normaya 1, 2, 3 , Hakimah Ismail 1 , Anwar Iqbal 4 , Bijarimi Mat Piah 5 , Mohd Armi Abu Samah 1, 2, 3 , Mohammad Norazmi Ahmad 1, 2, 3, 6
ACS Omega ( IF 3.7 ) Pub Date : 2021-09-21 , DOI: 10.1021/acsomega.1c02699 Muhammad Ammar Mohammad Alwi 1 , Erna Normaya 1, 2, 3 , Hakimah Ismail 1 , Anwar Iqbal 4 , Bijarimi Mat Piah 5 , Mohd Armi Abu Samah 1, 2, 3 , Mohammad Norazmi Ahmad 1, 2, 3, 6
Affiliation
|
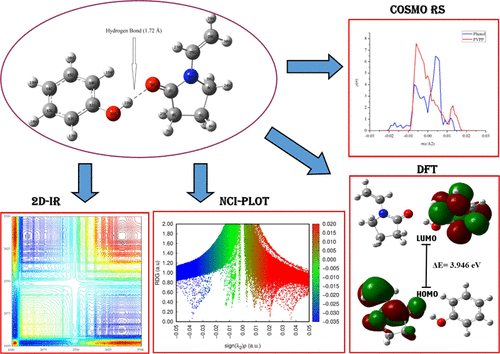
The discharge of industrial effluents, such as phenol, into aquatic and soil environments is a global problem due to its serious negative impacts on human health and aquatic ecosystems. In this study, the ability of polyvinylpolypyrrolidone (PVPP) to remove phenol from an aqueous medium was investigated. The results showed that a significant proportion of phenol (up to 74.91%) was removed using PVPP at pH 6.5. Isotherm adsorption experiments of phenol on PVPP indicated that the best-fit adsorption was obtained using Langmuir models. The response peaks of the hydroxyl groups of phenol (OH) and the carboxyl groups (i.e., C═O) of PVPP were altered, indicating the formation of a hydrogen bond between the PVPP and phenol during phenol removal, as characterized using 1D and 2D IR spectroscopy. The resulting complexes were successfully characterized based on their thermodynamic properties, Mulliken charge, and electronic transition using the DFT approach. To clarify the types of interactions taking place in the complex systems, quantum theory of atoms in molecules (QTAIM) analysis, reduced density gradient noncovalent interaction (RDG-NCI) approach, and conductor-like screening model for real solvents (COSMO-RS) approach were also successfully calculated. The results showed that the interactions that occurred in the process of removing phenol by PVPP were through hydrogen bonding (based on RDG-NCI and COSMO-RS), which was identified as an intermediate type (∇2ρ(r) > 0 and H < 0, QTAIM). To gain a deeper understanding of how these interactions occurred, further characterization was performed based on adsorption mechanisms using molecular electrostatic potential, global reactivity, and local reactivity descriptors. The results showed that during hydrogen bond formation, PVPP acts as a nucleophile, whereas phenol acts as an electrophile and the O9 atom (i.e., donor electron) reacts with the H22 atom (i.e., acceptor electron).
中文翻译:

聚乙烯聚吡咯烷酮在水性介质中有效去除苯酚的吸附机理的二维红外相关光谱、类导体筛选模型和密度泛函理论研究
由于苯酚等工业废水排放到水生和土壤环境中,对人类健康和水生生态系统造成严重的负面影响,因此是一个全球性问题。在这项研究中,研究了聚乙烯聚吡咯烷酮 (PVPP) 从水性介质中去除苯酚的能力。结果表明,使用 pH 为 6.5 的 PVPP 可以去除显着比例的苯酚(高达 74.91%)。苯酚在 PVPP 上的等温线吸附实验表明,使用朗缪尔模型获得了最佳吸附。苯酚(OH)的羟基和 PVPP 的羧基(即 C=O)的响应峰发生了改变,表明在苯酚去除过程中 PVPP 和苯酚之间形成了氢键,如使用 1D 和 2D 表征红外光谱。使用 DFT 方法基于热力学性质、马利肯电荷和电子跃迁成功表征了所得配合物。为了阐明复杂系统中发生的相互作用类型,分子中原子的量子理论 (QTAIM) 分析、降低密度梯度非共价相互作用 (RDG-NCI) 方法和真实溶剂的类导体筛选模型 (COSMO-RS)方法也被成功计算。结果表明,PVPP去除苯酚过程中发生的相互作用是通过氢键(基于RDG-NCI和COSMO-RS),被鉴定为中间型(∇2ρ(还成功计算了分子中原子的量子理论 (QTAIM) 分析、降低密度梯度非共价相互作用 (RDG-NCI) 方法和真实溶剂的类导体筛选模型 (COSMO-RS) 方法。结果表明,PVPP去除苯酚过程中发生的相互作用是通过氢键(基于RDG-NCI和COSMO-RS),被鉴定为中间型(∇2ρ(还成功计算了分子中原子的量子理论 (QTAIM) 分析、降低密度梯度非共价相互作用 (RDG-NCI) 方法和真实溶剂的类导体筛选模型 (COSMO-RS) 方法。结果表明,PVPP去除苯酚过程中发生的相互作用是通过氢键(基于RDG-NCI和COSMO-RS),被鉴定为中间型(∇2ρ(r ) > 0 和H < 0,QTAIM)。为了更深入地了解这些相互作用是如何发生的,使用分子静电势、全局反应性和局部反应性描述符基于吸附机制进行了进一步的表征。结果表明,在氢键形成过程中,PVPP充当亲核试剂,而苯酚充当亲电试剂,O9原子(即供体电子)与H22原子(即受体电子)反应。
更新日期:2021-10-06
中文翻译:

聚乙烯聚吡咯烷酮在水性介质中有效去除苯酚的吸附机理的二维红外相关光谱、类导体筛选模型和密度泛函理论研究
由于苯酚等工业废水排放到水生和土壤环境中,对人类健康和水生生态系统造成严重的负面影响,因此是一个全球性问题。在这项研究中,研究了聚乙烯聚吡咯烷酮 (PVPP) 从水性介质中去除苯酚的能力。结果表明,使用 pH 为 6.5 的 PVPP 可以去除显着比例的苯酚(高达 74.91%)。苯酚在 PVPP 上的等温线吸附实验表明,使用朗缪尔模型获得了最佳吸附。苯酚(OH)的羟基和 PVPP 的羧基(即 C=O)的响应峰发生了改变,表明在苯酚去除过程中 PVPP 和苯酚之间形成了氢键,如使用 1D 和 2D 表征红外光谱。使用 DFT 方法基于热力学性质、马利肯电荷和电子跃迁成功表征了所得配合物。为了阐明复杂系统中发生的相互作用类型,分子中原子的量子理论 (QTAIM) 分析、降低密度梯度非共价相互作用 (RDG-NCI) 方法和真实溶剂的类导体筛选模型 (COSMO-RS)方法也被成功计算。结果表明,PVPP去除苯酚过程中发生的相互作用是通过氢键(基于RDG-NCI和COSMO-RS),被鉴定为中间型(∇2ρ(还成功计算了分子中原子的量子理论 (QTAIM) 分析、降低密度梯度非共价相互作用 (RDG-NCI) 方法和真实溶剂的类导体筛选模型 (COSMO-RS) 方法。结果表明,PVPP去除苯酚过程中发生的相互作用是通过氢键(基于RDG-NCI和COSMO-RS),被鉴定为中间型(∇2ρ(还成功计算了分子中原子的量子理论 (QTAIM) 分析、降低密度梯度非共价相互作用 (RDG-NCI) 方法和真实溶剂的类导体筛选模型 (COSMO-RS) 方法。结果表明,PVPP去除苯酚过程中发生的相互作用是通过氢键(基于RDG-NCI和COSMO-RS),被鉴定为中间型(∇2ρ(r ) > 0 和H < 0,QTAIM)。为了更深入地了解这些相互作用是如何发生的,使用分子静电势、全局反应性和局部反应性描述符基于吸附机制进行了进一步的表征。结果表明,在氢键形成过程中,PVPP充当亲核试剂,而苯酚充当亲电试剂,O9原子(即供体电子)与H22原子(即受体电子)反应。









































 京公网安备 11010802027423号
京公网安备 11010802027423号