Biochimica et Biophysica Acta (BBA) - General Subjects ( IF 2.8 ) Pub Date : 2021-09-22 , DOI: 10.1016/j.bbagen.2021.130016 Aline Aparecida de Souza 1 , Débora Martins de Andrade 1 , Fábio da Silva Siqueira 1 , Juliana Fortes Di Iorio 1 , Marcia Paranho Veloso 2 , Camila de Morais Coelho 2 , Claudio Viegas Junior 3 , Vanessa Silva Gontijo 3 , Marcelo Henrique Dos Santos 4 , Maria Cecília Zorél Meneghetti 5 , Helena Bonciani Nader 5 , Ivarne Luis Dos Santos Tersariol 6 , Luiz Juliano 7 , Maria Aparecida Juliano 7 , Wagner Alves de Souza Judice 1
|
Background
Garcinia brasiliensis is a species native to the Amazon forest. The white mucilaginous pulp is used in folk medicine as a wound healing agent and for peptic ulcer, urinary, and tumor disease treatments. The activity of the proprotein convertases (PCs) Subtilisin/Kex is associated with the development of viral, bacterial and fungal infections, osteoporosis, hyperglycemia, atherosclerosis, cardiovascular, neurodegenerative and neoplastic diseases.
Methods
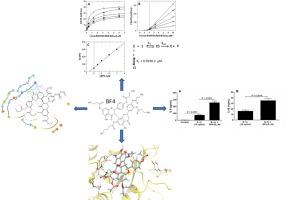
Morelloflavone (BF1) and semisynthetic biflavonoid (BF2, 3 and 4) from Garcinia brasiliensis were tested as inhibitor of PCs Kex2, PC1/3 and Furin, and determined IC50, Ki, human proinflammatory cytokines secretion in Caco-2 cells, mechanism of inhibition, and performed molecular docking studies.
Results
Biflavonoids were more effective in the inhibition of neuroendocrine PC1/3 than mammalian Furin and fungal Kex2. BF1 presented a mixed inhibition mechanism for Kex2 and PC1, and competitive inhibition for Furin. BF4 has no good interaction with Kex2 and Furin since carboxypropyl groups results in steric hindrance to ligand-protein interactions. Carboxypropyl groups of BF4 promote steric hindrance with Kex2 and Furin, but effective in the affinity of PC1/3. BF4 was more efficient at inhibiting PCl/3 (IC50 = 1.13 μM and Ki = 0,59 μM, simple linear competitive mechanism of inhibition) than Kex2, Furin. Also, our results strongly suggested that BF4 also inhibits the endogenous cellular PC1/3 activity in Caco-2 cells, since PC1/3 inhibition by BF4 causes a large increase in IL-8 and IL-1β secretion in Caco-2 cells.
Conclusions
BF4 is a potent and selective inhibitor of PC1/3.
General significance
BF4 is the best candidate for further clinical studies on inhibition of PC1/3.
中文翻译:

Semysinthetic 双黄酮类 Morelloflavone-7,4′,7″,3‴,4‴-penta-O-butanoyl 是比 Kex2 和 Furin 更有效的前蛋白转化酶 Subtilisin/Kexin PC1/3 抑制剂
背景
巴西藤黄 (Garcinia brasiliensis)是一种原产于亚马逊森林的物种。白色粘液浆在民间医学中用作伤口愈合剂以及消化性溃疡、泌尿系统疾病和肿瘤疾病的治疗。前蛋白转化酶 (PC) 枯草杆菌蛋白酶/Kex 的活性与病毒、细菌和真菌感染、骨质疏松症、高血糖、动脉粥样硬化、心血管、神经退行性和肿瘤疾病的发生有关。
方法
测试了来自巴西藤黄果的莫雷洛黄酮 (BF1) 和半合成双类黄酮 (BF2、3 和 4) 作为 PC Kex2、PC1/3 和 Furin 的抑制剂,并测定了 IC 50 、 K 、Caco-2 细胞中人类促炎细胞因子的分泌、机制抑制,并进行分子对接研究。
结果
双黄酮类化合物在抑制神经内分泌 PC1/3 方面比哺乳动物 Furin 和真菌 Kex2 更有效。 BF1 对 Kex2 和 PC1 呈现混合抑制机制,对 Furin 呈现竞争性抑制机制。 BF4 与 Kex2 和 Furin 没有良好的相互作用,因为羧丙基会导致配体-蛋白质相互作用的空间位阻。 BF4 的羧丙基可促进与 Kex2 和 Furin 的空间位阻,但对 PC1/3 的亲和力有效。 BF4 比 Kex2、弗林蛋白酶更有效地抑制 PCl/3(IC 50 = 1.13 μM 和K = 0,59 μM,简单的线性竞争抑制机制)。此外,我们的结果强烈表明,BF4 还抑制 Caco-2 细胞中的内源性细胞 PC1/3 活性,因为 BF4 抑制 PC1/3 会导致 Caco-2 细胞中 IL-8 和 IL-1β 分泌大量增加。
结论
BF4 是一种有效的、选择性的 PC1/3 抑制剂。
一般意义
BF4 是进一步抑制 PC1/3 临床研究的最佳候选者。











































 京公网安备 11010802027423号
京公网安备 11010802027423号