Journal of Allergy and Clinical Immunology ( IF 11.4 ) Pub Date : 2021-09-22 , DOI: 10.1016/j.jaci.2021.09.013 Vadim Pivniouk 1 , Joao A Gimenes-Junior 2 , Peace Ezeh 2 , Ashley Michael 2 , Oksana Pivniouk 2 , Seongmin Hahn 2 , Sydney R VanLinden 2 , Sean P Malone 2 , Amir Abidov 3 , Dayna Anderson 2 , Justyna Gozdz 2 , Avery DeVries 4 , Fernando D Martinez 4 , Christian Pasquali 5 , Donata Vercelli 6
|
Background
Microbial interventions against allergic asthma have robust epidemiologic underpinnings and the potential to recalibrate disease-inducing immune responses. Oral administration of OM-85, a standardized lysate of human airways bacteria, is widely used empirically to prevent respiratory infections and a clinical trial is testing its ability to prevent asthma in high-risk children. We previously showed that intranasal administration of microbial products from farm environments abrogates experimental allergic asthma.
Objectives
We sought to investigate whether direct administration of OM-85 to the airway compartment protects against experimental allergic asthma; and to identify protective cellular and molecular mechanisms activated through this natural route.
Methods
Different strains of mice sensitized and challenged with ovalbumin or Alternaria received OM-85 intranasally, and cardinal cellular and molecular asthma phenotypes were measured. Airway transfer experiments assessed whether OM-85–treated dendritic cells protect allergen-sensitized, OM-85–naive mice against asthma.
Results
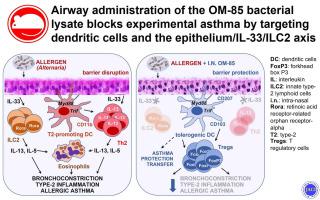
Airway OM-85 administration suppressed allergic asthma in all models acting on multiple innate and adaptive immune targets: the airway epithelium/IL-33/ILC2 axis, lung allergen–induced type 2 responses, and dendritic cells whose Myd88/Trif-dependent tolerogenic reprogramming was sufficient to transfer OM-85–induced asthma protection.
Conclusions
We provide the first demonstration that administering a standardized bacterial lysate to the airway compartment protects from experimental allergic asthma by engaging multiple immune pathways. Because protection required a cumulative dose 27- to 46-fold lower than the one reportedly active through the oral route, the efficacy of intranasal OM-85 administration may reflect its direct access to the airway mucosal networks controlling the initiation and development of allergic asthma.
中文翻译:

OM-85(一种细菌裂解物)的气道给药通过靶向树突状细胞和上皮/IL-33/ILC2 轴来阻断实验性哮喘
背景
针对过敏性哮喘的微生物干预具有强大的流行病学基础,并有可能重新调整诱发疾病的免疫反应。口服 OM-85 是一种标准化的人类呼吸道细菌裂解物,根据经验广泛用于预防呼吸道感染,一项临床试验正在测试其预防高危儿童哮喘的能力。我们之前表明,鼻内施用来自农场环境的微生物产品可以消除实验性过敏性哮喘。
目标
我们试图调查将 OM-85 直接给药至气道隔室是否可以预防实验性过敏性哮喘;并确定通过这种自然途径激活的保护性细胞和分子机制。
方法
用卵白蛋白或链格孢菌致敏和攻击的不同品系小鼠鼻内接受 OM-85,并测量了主要的细胞和分子哮喘表型。气道转移实验评估了经 OM-85 处理的树突状细胞是否能保护过敏原致敏的、未经 OM-85 处理的小鼠免受哮喘的侵害。
结果
气道 OM-85 给药抑制了所有模型中的过敏性哮喘,这些模型作用于多种先天性和适应性免疫靶点:气道上皮细胞/IL-33/ILC2 轴、肺过敏原诱导的 2 型反应,以及 Myd88/Trif 依赖性致耐受性重编程的树突状细胞足以转移 OM-85 诱导的哮喘保护作用。
结论
我们提供了第一个证明,即向气道室施用标准化的细菌裂解物可通过参与多种免疫途径来防止实验性过敏性哮喘。由于保护所需的累积剂量比据报道通过口服途径激活的剂量低 27 至 46 倍,因此鼻内 OM-85 给药的功效可能反映其直接进入控制过敏性哮喘的发生和发展的气道粘膜网络。











































 京公网安备 11010802027423号
京公网安备 11010802027423号