Journal of Molecular Liquids ( IF 5.3 ) Pub Date : 2021-09-21 , DOI: 10.1016/j.molliq.2021.117600 Lukman O. Olasunkanmi 1, 2 , Nancy I. Aniki 1, 2 , Abolanle S. Adekunle 1, 2 , Lateefa M. Durosinmi 1 , Solomon S. Durodola 1, 2 , Olaide O.Wahab 3 , Eno E. Ebenso 4
|
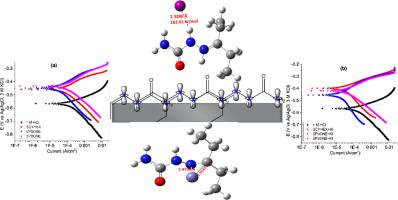
Three hydrazinecarboxamides, namely, 2-cyclohexylidenehydrazinecarboxamide (SCYHEX), 2-(pentan-3-ylidene)hydrazinecarboxamide (SP3ONE) and 2-(pentan-2-ylidene)hydrazinecarboxamide (SP2ONE) were tested as inhibitors of acid corrosion of mild steel both without and with addition of iodide ions. The formulated inhibitor solutions showed excellent corrosion inhibition efficiency as they suppressed steel to the tune of 82% without KI additive, and up to 97% with KI additive. The shifts in corrosion potentials recorded from polarization measurements were anodic and ranged from 25 mV to 55 mV (without KI) relative to the uninhibited electrolyte, suggesting that the Schiff bases are mixed-type corrosion inhibitors with prominent effects on anodic dissolution of steel. The inhibitors adsorb at steel/electrolyte interface as reveled by the AC impedance measurements that showed more than 5 times increase in the charge transfer resistance and equivalence decrease in double layer capacitance of the inhibitor-containing solutions compared to the blank. Theoretical density functional theory (DFT) calculations suggested possible covalent interactions between the Schiff bases molecules and Fe with Fe---N and Fe---C bond lengths ranging from 1.87 Å to 1.96 Å. Experimentally derived synergistic parameters for Schiff bases-iodide ions interactions were generally greater than 1 and a strong H---I bond of ca. 2.3 Å length was observed in what appeared to be first time theoretical modelling of inhibitor-iodide ion synergistic interactions. DFT derived reactivity indices of protonated Schiff bases molecules correlated with the observed strengths of corrosion inhibition. Monte Carlo simulations study also confirmed the tendency of the inhibitor molecules to adsorb on mild steel surface and displace corrosive ions from the steel surface.
中文翻译:

研究一些肼甲酰胺和碘离子在盐酸中作为低碳钢腐蚀抑制剂配方的协同作用:实验和计算研究
测试了三种肼甲酰胺,即 2-亚环己基肼甲酰胺 (SCYHEX)、2-(戊-3-亚基)肼甲酰胺 (SP3ONE) 和 2-(戊-2-亚基)肼甲酰胺 (SP2ONE) 作为低碳钢的酸腐蚀抑制剂。不加和加碘离子。配制的抑制剂溶液显示出优异的腐蚀抑制效率,因为它们在没有 KI 添加剂的情况下将钢抑制到 82%,而在使用 KI 添加剂的情况下抑制高达 97%。从极化测量记录的腐蚀电位的变化是阳极的,相对于未抑制的电解质,范围从 25 mV 到 55 mV(无 KI),这表明希夫碱是混合型腐蚀抑制剂,对钢的阳极溶解有显着影响。交流阻抗测量表明,抑制剂吸附在钢/电解质界面上,与空白相比,含抑制剂溶液的电荷转移电阻增加了 5 倍以上,双电层电容等效降低。理论密度泛函理论 (DFT) 计算表明,席夫碱分子与 Fe 之间可能存在共价相互作用,其中 Fe---N 和 Fe---C 键长范围为 1.87 Å 至 1.96 Å。席夫碱-碘离子相互作用的实验得出的协同参数通常大于 1,强 H---I 键约为 在抑制剂-碘离子协同相互作用的首次理论模型中观察到 2.3 Å 长度。DFT 衍生的质子化席夫碱分子的反应性指数与观察到的腐蚀抑制强度相关。蒙特卡罗模拟研究还证实了抑制剂分子吸附在低碳钢表面并从钢表面置换腐蚀性离子的趋势。











































 京公网安备 11010802027423号
京公网安备 11010802027423号