Journal of Membrane Science ( IF 8.4 ) Pub Date : 2021-09-21 , DOI: 10.1016/j.memsci.2021.119899 Arturo Ortega 1 , Luis F. Arenas 2 , Joep J.H. Pijpers 3 , Diana L. Vicencio 1 , Juan C. Martínez 1 , Francisca A. Rodríguez 1 , Eligio P. Rivero 1
|
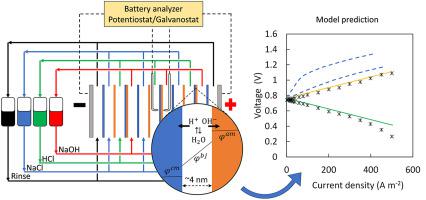
Research on flow batteries based on water dissociation and acid-base neutralization reactions at bipolar membranes is driven by the possibility of a low-cost and environmentally friendly technology. However, their application in energy storage requires a high round-trip efficiency, which has yet to be realized. In order to establish which critical factors determine their efficiency, this work examines the distribution of potential and concentration in a laboratory scale acid-base flow battery by using fundamental models. Transport mechanisms of diffusion, convection and migration were incorporated into the Nernst-Planck equation. Water dissociation during the charging step was modeled by the second Wien effect combined with the catalytic effect produced by functional groups or by catalysts present in the bipolar junction and compared to the water dissociation equilibrium model. The discharge was modeled by neutralization reaction kinetics and was also compared to the equilibrium model. All model parameters were firmly established and were determined or estimated from information available from the membrane supplier or the literature. The current-potential behavior predicted by the model for both charge and discharge closely matches experimental data and provides a lead for future work on full-scale modeling of acid-base flow batteries.
中文翻译:

模拟酸碱液流电池双极膜中的水离解、酸碱中和和离子传输
基于双极膜水离解和酸碱中和反应的液流电池研究受到低成本和环保技术的推动。然而,它们在储能方面的应用需要很高的往返效率,这一点尚未实现。为了确定哪些关键因素决定了它们的效率,这项工作通过使用基本模型检查了实验室规模酸碱液流电池中电位和浓度的分布。扩散、对流和迁移的传输机制被纳入 Nernst-Planck 方程。充电步骤期间的水离解通过第二维恩效应与官能团或双极结中存在的催化剂产生的催化作用相结合,并与水离解平衡模型进行比较。放电通过中和反应动力学建模,并与平衡模型进行比较。所有模型参数均已确定并根据膜供应商或文献中提供的信息确定或估计。该模型预测的充电和放电电流电位行为与实验数据密切匹配,并为未来酸碱液流电池全尺寸建模的工作提供了线索。所有模型参数均已确定并根据膜供应商或文献中提供的信息确定或估计。该模型预测的充电和放电电流电位行为与实验数据密切匹配,并为未来酸碱液流电池全尺寸建模的工作提供了线索。所有模型参数均已确定并根据膜供应商或文献中提供的信息确定或估计。该模型预测的充电和放电电流电位行为与实验数据密切匹配,并为未来酸碱液流电池全尺寸建模的工作提供了线索。











































 京公网安备 11010802027423号
京公网安备 11010802027423号