Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2021-09-21 , DOI: 10.1016/j.jhazmat.2021.127321 Zhiping Ye 1 , Guanjie Wang 1 , Jean-Marc Giraudon 2 , Anton Nikiforov 3 , Jun Chen 4 , Liang Zhao 1 , Xiuwen Zhang 1 , Jiade Wang 1
|
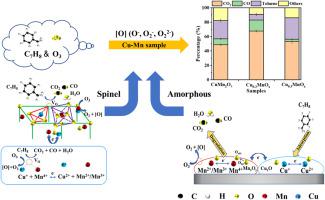
The effect of different crystal phases, i.e. spinel phase (CuMn2O4) and amorphous phase (Cu0.2MnOx), was explored in Cu-Mn catalytic ozonation of toluene. The toluene removal efficiency followed the order of Cu0.2MnOx (91.2%) ˃ CuMn2O4 (74.5%) ˃ commercial catalyst Cu0.3MnOx (70.3%) in 130 min, and the higher CO2 yield (67.6%) could be also observed using Cu0.2MnOx. In order to investigate the effect of phases on the toluene degradation pathway, the intermediates and byproducts were identified by DRIFTS, GC-MS, and TOF-SIMS. No obvious difference was observed in the distribution of byproducts, except for the quantities, suggesting the discrepancy of oxidation rate. On the other hand, the catalysts were characterized before and after the ozonation process by TEM, BET, XPS, XRD, EPR, TGA, and TPR. It was proposed that for amorphous catalysts, the oxygen vacancy (Vo) helped the chemisorption of toluene, and adjacent Mn reacted as the main active site for the ozonation process. While, the redox pair of Cu+/Mn4+ and Cu2+/(Mn3+, Mn2+) in the spinel phase plays an important role in the generation of oxygen vacancies for O3 decomposition.
中文翻译:

Cu-Mn催化甲苯臭氧化的研究:晶相、中间体和机理
探讨了不同晶相,即尖晶石相(CuMn 2 O 4)和非晶相(Cu 0.2 MnO x)在Cu-Mn催化臭氧化甲苯中的作用。130 min内甲苯去除率依次为Cu 0.2 MnO x (91.2%)˃CuMn 2 O 4 (74.5%)˃商业催化剂Cu 0.3 MnO x (70.3%),CO 2收率较高(67.6%)也可以使用 Cu 0.2 MnO x观察到. 为了研究相对甲苯降解途径的影响,通过 DRIFTS、GC-MS 和 TOF-SIMS 鉴定了中间体和副产物。除数量外,副产物的分布没有明显差异,表明氧化速率存在差异。另一方面,通过 TEM、BET、XPS、XRD、EPR、TGA 和 TPR 对臭氧化过程前后的催化剂进行了表征。有人提出,对于无定形催化剂,氧空位(V o)有助于甲苯的化学吸附,相邻的 Mn 作为臭氧化过程的主要活性位点发生反应。而Cu + /Mn 4+和Cu 2+ /(Mn 3+ , Mn 2+的氧化还原对) 在尖晶石相中,对于 O 3分解的氧空位的产生起着重要的作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号