Journal of the Energy Institute ( IF 5.6 ) Pub Date : 2021-09-20 , DOI: 10.1016/j.joei.2021.09.007 Akihiro Ueda 1 , Keiya Nisida 1 , Yukihiko Matsumura 1 , Takayuki Ichikawa 1 , Yutaka Nakashimada 1 , Takuma Endo 1 , Wookyung Kim 1
|
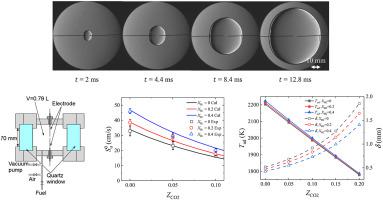
The effects of different mole fractions of hydrogen and carbon dioxide on the combustion characteristics of a premixed methane–air mixture are experimentally and numerically investigated. The laminar burning velocity of hydrogen-methane-carbon dioxide-air mixture was measured using the spherically expanding flame method at the initial temperature and pressure of 283 K and 0.1 MPa, respectively. Additionally, numerical analysis is conducted under steady 1D laminar flow conditions to investigate the adiabatic flame temperature, dominant elementary reactions, and NO formation. The measured velocities correspond with those estimated numerically. The results show that increasing the carbon dioxide mole fraction decreases the laminar burning velocity, attributed to the carbon dioxide dilution, which decreases the thermal diffusivity and flame temperature. Conversely, the velocity increases with the thermal diffusivity as the hydrogen mole fraction increases. Moreover, the hydrogen addition leads to chain-branching reactions that produce active H, O, and OH radicals via the oxidation of hydrocarbons, which is the rate-determining reaction. Furthermore, an increase in the mole fractions of hydrogen and carbon dioxide decreases the NO production amount.
中文翻译:

氢气和二氧化碳对甲烷-空气混合物层流燃烧速度的影响
通过实验和数值研究了不同摩尔分数的氢气和二氧化碳对预混甲烷-空气混合物燃烧特性的影响。在初始温度和压力分别为 283 K 和 0.1 MPa 下,使用球形膨胀火焰法测量了氢气-甲烷-二氧化碳-空气混合物的层流燃烧速度。此外,数值分析是在稳定的一维层流条件下进行的,以研究绝热火焰温度、主要元素反应和 NO 形成。测得的速度与数值估计的速度相对应。结果表明,由于二氧化碳稀释,增加二氧化碳摩尔分数会降低层流燃烧速度,从而降低热扩散率和火焰温度。相反,随着氢摩尔分数的增加,速度随着热扩散率的增加而增加。此外,氢加成导致链支化反应,通过碳氢化合物的氧化产生活性 H、O 和 OH 自由基,这是限速反应。此外,氢气和二氧化碳的摩尔分数增加会降低 NO 的生成量。











































 京公网安备 11010802027423号
京公网安备 11010802027423号