当前位置:
X-MOL 学术
›
ACS Synth. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Engineering T4 Bacteriophage for In Vivo Display by Type V CRISPR-Cas Genome Editing
ACS Synthetic Biology ( IF 3.7 ) Pub Date : 2021-09-21 , DOI: 10.1021/acssynbio.1c00251 Junhua Dong 1, 2, 3 , Cen Chen 1, 2, 3 , Yuepeng Liu 1, 2, 3 , Jingen Zhu 4 , Mengling Li 1, 2, 3 , Venigalla B Rao 4 , Pan Tao 1, 2, 3
ACS Synthetic Biology ( IF 3.7 ) Pub Date : 2021-09-21 , DOI: 10.1021/acssynbio.1c00251 Junhua Dong 1, 2, 3 , Cen Chen 1, 2, 3 , Yuepeng Liu 1, 2, 3 , Jingen Zhu 4 , Mengling Li 1, 2, 3 , Venigalla B Rao 4 , Pan Tao 1, 2, 3
Affiliation
|
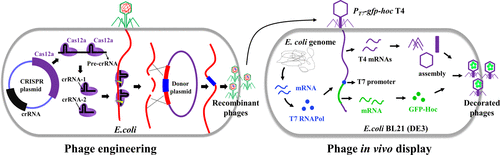
Bacteriophage T4 has enormous potential for biomedical applications due to its large size, capsid architecture, and high payload capability for protein and DNA delivery. However, it is not very easy to genetically engineer its genome heavily modified by cytosine hydroxymethylation and glucosylation. The glucosyl hydroxymethyl cytosine (ghmC) genome of phage is completely resistant to most restriction endonucleases and exhibits various degrees of resistance to CRISPR-Cas systems. Here, we found that the type V CRISPR-Cas12a system, which shows efficient cleavage of ghmC-modified genome when compared to the type II CRISPR-Cas9 system, can be synergistically employed to generate recombinant T4 phages. Focused on surface display, we analyzed the ability of phage T4 outer capsid proteins Hoc (highly antigenic outer capsid protein) and Soc (small outer capsid protein) to tether, in vivo, foreign peptides and proteins to T4 capsid. Our data show that while these could be successfully expressed and displayed during the phage infection, shorter peptides are present at a much higher copy number than full-length proteins. However, the copy number of the latter could be elevated by driving the expression of the transgene using the strong T7 RNA polymerase expression system. This CRISPR-inspired approach has the potential to expand the application of phages to various basic and translational research projects.
中文翻译:

通过 V 型 CRISPR-Cas 基因组编辑工程 T4 噬菌体用于体内展示
噬菌体 T4 由于其大尺寸、衣壳结构以及蛋白质和 DNA 递送的高有效负载能力,在生物医学应用方面具有巨大潜力。然而,通过胞嘧啶羟甲基化和糖基化对其基因组进行基因工程改造并不容易。噬菌体的葡萄糖基羟甲基胞嘧啶 (ghmC) 基因组对大多数限制性内切酶具有完全抗性,并对 CRISPR-Cas 系统表现出不同程度的抗性。在这里,我们发现,与 II 型 CRISPR-Cas9 系统相比,V 型 CRISPR-Cas12a 系统显示出对 ghmC 修饰的基因组的有效切割,可以协同使用来生成重组 T4 噬菌体。重点关注表面展示,我们分析了噬菌体 T4 外衣壳蛋白 Hoc(高抗原性外衣壳蛋白)和 Soc(小外衣壳蛋白)在体内将外源肽和蛋白质束缚到 T4 衣壳的能力。我们的数据表明,虽然这些可以在噬菌体感染期间成功表达和展示,但较短的肽以比全长蛋白质高得多的拷贝数存在。然而,后者的拷贝数可以通过使用强T7 RNA聚合酶表达系统驱动转基因的表达来提高。这种受 CRISPR 启发的方法有可能将噬菌体的应用扩展到各种基础和转化研究项目。
更新日期:2021-10-15
中文翻译:

通过 V 型 CRISPR-Cas 基因组编辑工程 T4 噬菌体用于体内展示
噬菌体 T4 由于其大尺寸、衣壳结构以及蛋白质和 DNA 递送的高有效负载能力,在生物医学应用方面具有巨大潜力。然而,通过胞嘧啶羟甲基化和糖基化对其基因组进行基因工程改造并不容易。噬菌体的葡萄糖基羟甲基胞嘧啶 (ghmC) 基因组对大多数限制性内切酶具有完全抗性,并对 CRISPR-Cas 系统表现出不同程度的抗性。在这里,我们发现,与 II 型 CRISPR-Cas9 系统相比,V 型 CRISPR-Cas12a 系统显示出对 ghmC 修饰的基因组的有效切割,可以协同使用来生成重组 T4 噬菌体。重点关注表面展示,我们分析了噬菌体 T4 外衣壳蛋白 Hoc(高抗原性外衣壳蛋白)和 Soc(小外衣壳蛋白)在体内将外源肽和蛋白质束缚到 T4 衣壳的能力。我们的数据表明,虽然这些可以在噬菌体感染期间成功表达和展示,但较短的肽以比全长蛋白质高得多的拷贝数存在。然而,后者的拷贝数可以通过使用强T7 RNA聚合酶表达系统驱动转基因的表达来提高。这种受 CRISPR 启发的方法有可能将噬菌体的应用扩展到各种基础和转化研究项目。











































 京公网安备 11010802027423号
京公网安备 11010802027423号