Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Quantum Chemical Calculation of the Effects of H2O on Oxygen Functional Groups during Coal Spontaneous Combustion
ACS Omega ( IF 4.1 ) Pub Date : 2021-09-20 , DOI: 10.1021/acsomega.1c03673 Yujia Huo 1 , Hongqing Zhu 1 , Xin He 1 , Shuhao Fang 1 , Wei Wang 1
ACS Omega ( IF 4.1 ) Pub Date : 2021-09-20 , DOI: 10.1021/acsomega.1c03673 Yujia Huo 1 , Hongqing Zhu 1 , Xin He 1 , Shuhao Fang 1 , Wei Wang 1
Affiliation
|
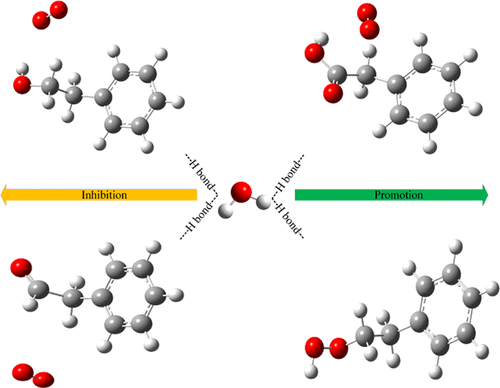
The effects of H2O on the low-temperature oxidation characteristics of coal have always been one of the keys in the research of coal spontaneous combustion, but most studies rely on experiments for macroscopic derivation, and theoretical researches at the microlevel are rarely mentioned. In this paper, phenylacetaldehyde, phenylethyl alcohol, phenylacetic acid, and ethylbenzene hydroperoxide were used as modeling compounds of coal molecules containing aldehyde (−CHO), alcohol hydroxyl (−OH), carboxyl (−COOH), and peroxide (−C–O–OH). The surface electrostatic potential (ESP), electron density of atoms in molecules (AIM), and reduced density gradient (RDG) of coal molecules were calculated by density functional theory (DFT), and the thermokinetic parameters of low-temperature oxidation of coal molecules with or without H2O were analyzed. The results show that the extreme positive and negative ESPs are located at the H and O atoms of oxygen functional groups (OFGs), respectively, which are the active sites for H2O adsorption. The AIM and RDG show that the phenylacetaldehyde···H2O complexes have two kinds of adsorption configurations with two and three hydrogen bonds, and that the phenylethyl alcohol···H2O complexes also have two kinds of adsorption configurations with one and three hydrogen bonds, and that both phenylacetic acid···H2O and ethylbenzene hydroperoxide···H2O only have one adsorption configuration, forming two and three hydrogen bonds, respectively. According to electron density ρ(r) and potential energy density V(r), the adsorption strength of H2O by four kinds of oxygen functional groups is ranked as −C–O–OH > −COOH > −OH > −CHO. The thermokinetic parameters show that H2O can increase the activation energy (ΔE) of the oxidation reactions of phenylacetaldehyde and phenylethyl alcohol, which can inhibit the reaction and decrease the activation energy (ΔE) of the oxidation reaction of phenylacetic acid and ethylbenzene hydroperoxide, which can promote the reactions.
中文翻译:

煤自燃过程中 H2O 对氧官能团影响的量子化学计算
H 2的影响O对煤的低温氧化特性一直是煤自燃研究的重点之一,但大部分研究依赖于宏观推导实验,微观层面的理论研究鲜有提及。在本文中,苯乙醛、苯乙醇、苯乙酸和乙苯氢过氧化物被用作含有醛 (-CHO)、醇羟基 (-OH)、羧基 (-COOH) 和过氧化物 (-C-O) 的煤分子的模拟化合物。 -哦)。利用密度泛函理论(DFT)计算了煤分子的表面静电势(ESP)、分子中原子的电子密度(AIM)、还原密度梯度(RDG),以及煤分子低温氧化的热力学参数有或没有 H 2O进行了分析。结果表明,极端正负ESP分别位于氧官能团(OFG)的H和O原子处,它们是H 2 O吸附的活性位点。AIM和RDG表明,苯乙醛...H 2 O配合物具有两个和三个氢键的两种吸附构型,苯乙醇...H 2 O配合物也有两种吸附构型,一种和三个氢键。三个氢键,苯乙酸...H 2 O 和乙苯氢过氧化物...H 2 O 只有一种吸附构型,分别形成两个和三个氢键。根据电子密度ρ( r) 和势能密度V ( r ),四种氧官能团对 H 2 O的吸附强度排序为 -C-O-OH > -COOH > -OH > -CHO。热力学参数表明,H 2 O可以提高苯乙醛和苯乙醇氧化反应的活化能(Δ E ),从而抑制反应,降低苯乙酸和乙苯氧化反应的活化能(Δ E )氢过氧化物,可以促进反应。
更新日期:2021-10-06
中文翻译:

煤自燃过程中 H2O 对氧官能团影响的量子化学计算
H 2的影响O对煤的低温氧化特性一直是煤自燃研究的重点之一,但大部分研究依赖于宏观推导实验,微观层面的理论研究鲜有提及。在本文中,苯乙醛、苯乙醇、苯乙酸和乙苯氢过氧化物被用作含有醛 (-CHO)、醇羟基 (-OH)、羧基 (-COOH) 和过氧化物 (-C-O) 的煤分子的模拟化合物。 -哦)。利用密度泛函理论(DFT)计算了煤分子的表面静电势(ESP)、分子中原子的电子密度(AIM)、还原密度梯度(RDG),以及煤分子低温氧化的热力学参数有或没有 H 2O进行了分析。结果表明,极端正负ESP分别位于氧官能团(OFG)的H和O原子处,它们是H 2 O吸附的活性位点。AIM和RDG表明,苯乙醛...H 2 O配合物具有两个和三个氢键的两种吸附构型,苯乙醇...H 2 O配合物也有两种吸附构型,一种和三个氢键。三个氢键,苯乙酸...H 2 O 和乙苯氢过氧化物...H 2 O 只有一种吸附构型,分别形成两个和三个氢键。根据电子密度ρ( r) 和势能密度V ( r ),四种氧官能团对 H 2 O的吸附强度排序为 -C-O-OH > -COOH > -OH > -CHO。热力学参数表明,H 2 O可以提高苯乙醛和苯乙醇氧化反应的活化能(Δ E ),从而抑制反应,降低苯乙酸和乙苯氧化反应的活化能(Δ E )氢过氧化物,可以促进反应。



























 京公网安备 11010802027423号
京公网安备 11010802027423号