当前位置:
X-MOL 学术
›
Acta Cryst. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A new guaninate hydrate K+·C5H4N5O−·H2O: crystal structure from 100 to 300 K in a comparison with 2Na+·C5H3N5O2−·7H2O
Acta Crystallographica Section B ( IF 1.3 ) Pub Date : 2021-09-21 , DOI: 10.1107/s205252062100754x Anna A. Gaydamaka , Sergey G. Arkhipov , Elena V. Boldyreva
Acta Crystallographica Section B ( IF 1.3 ) Pub Date : 2021-09-21 , DOI: 10.1107/s205252062100754x Anna A. Gaydamaka , Sergey G. Arkhipov , Elena V. Boldyreva
|
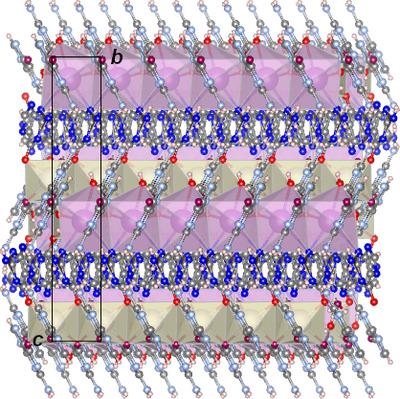
A new guanine salt hydrate, K+·C5H4N5O−·H2O, was obtained and characterized by single-crystal X-ray diffraction in the temperature range 100 K–300 K and compared with that of the previously documented sodium salt hydrate (2Na+·C5H3N5O2−·7H2O) [Gur & Shimon (2015). Acta Cryst. E71, 281–283; Gaydamaka et al. (2019). CrystEngComm, 21, 4484–4492]. Both sodium and potassium salt hydrates have channels. However, the structure of the channels, the cation coordination, the protonation (and, respectively, the charge) of the guanine anions, as well as the role of water molecules in the crystal structure are different for the two salt hydrates. In the crystal structures of the potassium salt, the guanine anions are linked via hydrogen bonds into quartets that form open cylindrical channels in a honeycomb framework. Water molecules `line the walls' of the channels, whereas the potassium cations fill the intra-channel space. This contrasts with the structure of the sodium salt hydrate in which guanine anions form channels with water molecules filling in the channel space together with sodium cations coordinating them. The 1D anionic assembly generated through numerous hydrogen bonds and cation interactions with guanine anions and water molecules is energetically the most distinctive part of the structure of the potassium salt hydrate. In the case of the guanine sodium salt, the structure contains purely inorganic polymeric fragments – sodium cations coordinated to a water molecule forming a 1D polymeric structure and guanine anions interconnecting these polymers via hydrogen bonds with water molecules. The structural differences account for the difference in the anisotropy of strain on temperature variation for the two salt hydrates: whereas in both structures the values of the bulk thermal expansion coefficients are similar in the two structures and the major expansion is observed along the channel axes, the degree of anisotropy for the K salt is more than four times higher than that for the Na salt.
中文翻译:

一种新的鸟嘌呤水合物 K+·C5H4N5O−·H2O:与 2Na+·C5H3N5O2−·7H2O 相比,100 到 300 K 的晶体结构
获得了一种新的鸟嘌呤盐水合物 K + ·C 5 H 4 N 5 O - ·H 2 O,并在 100 K-300 K 的温度范围内进行了单晶 X 射线衍射表征,并与之前的水合物进行了比较。记录在案的钠盐水合物 (2Na + ·C 5 H 3 N 5 O 2− ·7H 2 O) [Gur & Shimon (2015)。晶体学报。E 71 , 281–283; 盖达马卡等人。(2019)。CrystEngComm , 21, 4484–4492]。钠和钾的盐水合物都有通道。然而,两种盐水合物的通道结构、阳离子配位、鸟嘌呤阴离子的质子化(和电荷)以及水分子在晶体结构中的作用是不同的。在钾盐的晶体结构中,鸟嘌呤阴离子通过氢键形成四重体,在蜂窝框架中形成开放的圆柱形通道。水分子“排列在通道壁上”,而钾离子填充通道内空间。这与钠盐水合物的结构形成对比,其中鸟嘌呤阴离子形成通道,水分子与钠阳离子一起填充通道空间。通过大量氢键和阳离子与鸟嘌呤阴离子和水分子的相互作用产生的一维阴离子组装在能量上是钾盐水合物结构中最独特的部分。在鸟嘌呤钠盐的情况下,该结构包含纯无机聚合物片段——钠阳离子与水分子配位,形成一维聚合物结构,鸟嘌呤阴离子通过与水分子的氢键将这些聚合物互连。结构差异解释了两种盐水合物温度变化应变各向异性的差异:而在两种结构中,两种结构的整体热膨胀系数值相似,并且沿通道轴观察到主要膨胀, K盐的各向异性程度是Na盐的四倍多。
更新日期:2021-10-06
中文翻译:

一种新的鸟嘌呤水合物 K+·C5H4N5O−·H2O:与 2Na+·C5H3N5O2−·7H2O 相比,100 到 300 K 的晶体结构
获得了一种新的鸟嘌呤盐水合物 K + ·C 5 H 4 N 5 O - ·H 2 O,并在 100 K-300 K 的温度范围内进行了单晶 X 射线衍射表征,并与之前的水合物进行了比较。记录在案的钠盐水合物 (2Na + ·C 5 H 3 N 5 O 2− ·7H 2 O) [Gur & Shimon (2015)。晶体学报。E 71 , 281–283; 盖达马卡等人。(2019)。CrystEngComm , 21, 4484–4492]。钠和钾的盐水合物都有通道。然而,两种盐水合物的通道结构、阳离子配位、鸟嘌呤阴离子的质子化(和电荷)以及水分子在晶体结构中的作用是不同的。在钾盐的晶体结构中,鸟嘌呤阴离子通过氢键形成四重体,在蜂窝框架中形成开放的圆柱形通道。水分子“排列在通道壁上”,而钾离子填充通道内空间。这与钠盐水合物的结构形成对比,其中鸟嘌呤阴离子形成通道,水分子与钠阳离子一起填充通道空间。通过大量氢键和阳离子与鸟嘌呤阴离子和水分子的相互作用产生的一维阴离子组装在能量上是钾盐水合物结构中最独特的部分。在鸟嘌呤钠盐的情况下,该结构包含纯无机聚合物片段——钠阳离子与水分子配位,形成一维聚合物结构,鸟嘌呤阴离子通过与水分子的氢键将这些聚合物互连。结构差异解释了两种盐水合物温度变化应变各向异性的差异:而在两种结构中,两种结构的整体热膨胀系数值相似,并且沿通道轴观察到主要膨胀, K盐的各向异性程度是Na盐的四倍多。











































 京公网安备 11010802027423号
京公网安备 11010802027423号