International Journal of Biological Macromolecules ( IF 8.2 ) Pub Date : 2021-09-20 , DOI: 10.1016/j.ijbiomac.2021.09.083 Rafael J Borges 1 , Guilherme H M Salvador 2 , Henrique B Campanelli 2 , Daniel C Pimenta 3 , Mario de Oliveira Neto 2 , Isabel Usón 4 , Marcos R M Fontes 2
|
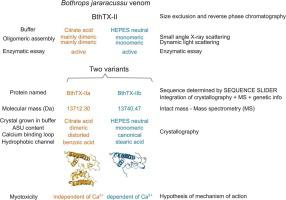
Phospholipases A2 (PLA2s) are found in almost every venomous snake family. In snakebites, some PLA2s can quickly cause local myonecrosis, which may lead to permanent sequelae if antivenom is administered belatedly. They hydrolyse phospholipids in membranes through a catalytic calcium ions-dependent mechanism. BthTX-II is a basic PLA2 and the second major component in the venom of Bothrops jararacussu. Herein, using the software SEQUENCE SLIDER, which integrates crystallographic, mass spectrometry and genetic data, we characterized the primary, tertiary and quaternary structure of two BthTX-II variants (called a and b), which diverge in 7 residues. Crystallographic structure BthTX-IIa is in a Tense-state with its distorted calcium binding loop buried in the dimer interface, contrarily, the novel BthTX-IIb structure is a monomer in a Relax-state with a fatty acid in the hydrophobic channel. Structural data in solution reveals that both variants are monomeric in neutral physiological conditions and mostly dimeric in an acidic environment, being catalytic active in both situations. Therefore, we propose two myotoxic mechanisms for BthTX-II, a catalytic one associated with the monomeric assembly, whereas the other has a calcium independent activity related to its C-terminal region, adopting a dimeric conformation similar to PLA2-like proteins.
中文翻译:

来自 Bothrops jararacussu 毒液的 BthTX-II 具有不同寡聚体组装的变体:蛇毒磷脂酶 A2 多功能性的一个例子
磷脂酶 A 2 (PLA 2 s) 几乎存在于每个毒蛇科中。在蛇咬伤中,一些 PLA 2 s 会迅速引起局部肌坏死,如果抗蛇毒血清给药较晚,可能会导致永久性后遗症。它们通过催化钙离子依赖性机制水解膜中的磷脂。BthTX-II 是一种基本的 PLA 2,是双龙鱼毒液中的第二个主要成分. 在本文中,我们使用集成了晶体学、质谱和遗传数据的软件 SEQUENCE SLIDER,表征了两个 BthTX-II 变体(称为 a 和 b)的一级、三级和四级结构,它们在 7 个残基中存在差异。晶体结构 BthTX-IIa 处于紧张状态,其扭曲的钙结合环埋在二聚体界面中,相反,新的 BthTX-IIb 结构是处于松弛状态的单体,在疏水通道中带有脂肪酸。溶液中的结构数据表明,两种变体在中性生理条件下都是单体,在酸性环境中主要是二聚体,在两种情况下都具有催化活性。因此,我们提出了 BthTX-II 的两种肌毒性机制,一种与单体组装相关的催化机制,2-样蛋白质。



























 京公网安备 11010802027423号
京公网安备 11010802027423号