Ceramics International ( IF 5.1 ) Pub Date : 2021-09-21 , DOI: 10.1016/j.ceramint.2021.09.203 Sultan Khan 1, 2 , Amarnath R. Allu 2, 3 , Anuraag Gaddam 4 , Hugo R. Fernandes 4 , Sutanu Dutta 1 , Partha S. Kongar 1 , Anal Tarafder 1, 2 , José M.F. Ferreira 4 , K. Annapurna 1, 2
|
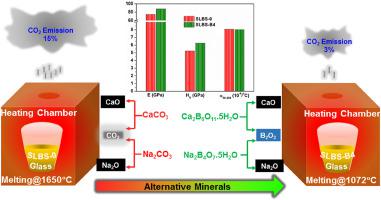
In view of recent energy crisis and environmental considerations, reductions of melting temperature and CO2 emissions during the production of commercial soda lime silicate (SLS) glass at industrial scale are major intentions. Here we show that this goal can be achieved by using carbonate-free borate minerals such as colemanite (Ca2B6O11•5H2O) and borax penta-hydrate (BPH, Na2B4O7•5H2O) as alternative sources for CaO and Na2O, respectively. The SLS glass melting temperature has decreased successively by the incorporation of B2O3 with substitution of equivalent wt% SiO2 resulting from gradual utilization of colemanite and BPH. Hot Stage Microscopy (HSM) revealed that the melting temperature was decreased from 1650 °C, for the parent commercial SLS glass, to 1528 °C on use of colemanite and further down to 1072 °C on utilization of both colemanite and appropriate quantity of BPH up to which the properties of the pristine glass can be retained. Thus, energy consumption equivalent to reduction in melting temperature could be minimized during the glass production. Another important environmental benefit achieved by using carbonate free minerals is significant reduction (12%) of CO2 emissions during thermal decomposition in glass melting which could minimize greenhouse gas to the atmosphere at a great extent. Further, the addition of colemanite improved micro-hardness, elastic modulus and thermal properties of the pristine glass through polymerization of glass network structure. In contrast to the use of BPH as a source of more than 8 wt.% Na2O deteriorated glass properties with the formation of non-bridging oxygens into the glass network.
中文翻译:

硬硼酸钙石和五水硼砂在钠钙硅酸盐玻璃熔炼中的应用——降低能耗和改善玻璃性能的策略
鉴于最近的能源危机和环境考虑,在工业规模的商业钠钙硅酸盐 (SLS) 玻璃生产过程中降低熔化温度和 CO 2排放是主要意图。在这里,我们表明可以通过使用不含碳酸盐的硼酸盐矿物,例如硬硼酸钙石(Ca 2 B 6 O 11 •5H 2 O)和五水硼砂(BPH,Na 2 B 4 O 7 •5H 2 O)来实现这一目标分别作为 CaO 和 Na 2 O 的替代来源。B 2 O 3的掺入使 SLS 玻璃的熔化温度连续下降随着硬硼酸钙石和 BPH 的逐渐利用而产生的等效重量百分比 SiO 2 的替代。热台显微镜 (HSM) 显示,使用硬硼酸钙石时,熔融温度从商业 SLS 母体玻璃的 1650°C 降至 1528°C,并在同时使用硬硼酸钙石和适量 BPH 时进一步降至 1072°C可以保留原始玻璃的特性。因此,在玻璃生产过程中,相当于降低熔化温度的能量消耗可以最小化。使用无碳酸盐矿物实现的另一个重要环境效益是显着减少 (12%) CO 2玻璃熔化过程中的热分解过程中的排放,这可以在很大程度上减少排放到大气中的温室气体。此外,硬硼酸钙石的添加通过玻璃网络结构的聚合提高了原始玻璃的显微硬度、弹性模量和热性能。与使用 BPH 作为超过 8 wt.% Na 2 O的来源相比,玻璃性能恶化,非桥接氧形成到玻璃网络中。











































 京公网安备 11010802027423号
京公网安备 11010802027423号