Cell Reports ( IF 7.5 ) Pub Date : 2021-09-21 , DOI: 10.1016/j.celrep.2021.109731 Zhenzhen Yan 1 , Haifeng Wu 1 , Hansen Liu 1 , Guimin Zhao 1 , Honghai Zhang 1 , Wanxin Zhuang 1 , Feng Liu 1 , Yi Zheng 1 , Bingyu Liu 1 , Lei Zhang 1 , Chengjiang Gao 1
|
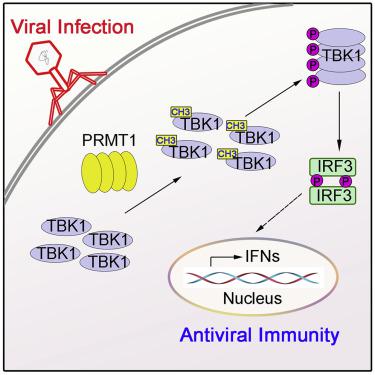
TBK1 is an essential kinase for the innate immune response against viral infection. However, the key molecular mechanisms regulating the TBK1 activation remain elusive. Here, we identify PRMT1, a type I protein arginine methyltransferase, as an essential regulator of TBK1 activation. PRMT1 directly interacts with TBK1 and catalyzes asymmetric methylation of R54, R134, and R228 on TBK1. This modification enhances TBK1 oligomerization after viral infection, which subsequently promotes TBK1 phosphorylation and downstream type I interferon production. More important, myeloid-specific Prmt1 knockout mice are more susceptible to infection with DNA and RNA viruses than Prmt1fl/fl mice. Our findings reveal insights into the molecular regulation of TBK1 activation and demonstrate the essential function of protein arginine methylation in innate antiviral immunity.
中文翻译:

蛋白质精氨酸甲基转移酶 PRMT1 通过不对称精氨酸甲基化促进 TBK1 活化
TBK1 是针对病毒感染的先天免疫反应的必需激酶。然而,调节 TBK1 激活的关键分子机制仍然难以捉摸。在这里,我们将 PRMT1(一种 I 型蛋白精氨酸甲基转移酶)鉴定为 TBK1 激活的重要调节因子。PRMT1 直接与 TBK1 相互作用并催化 TBK1 上 R54、R134 和 R228 的不对称甲基化。这种修饰增强了病毒感染后的 TBK1 寡聚化,从而促进了 TBK1 磷酸化和下游 I 型干扰素的产生。更重要的是,骨髓特异性Prmt1敲除小鼠比Prmt1 fl/fl更容易感染 DNA 和 RNA 病毒老鼠。我们的研究结果揭示了对 TBK1 激活的分子调控的见解,并证明了蛋白质精氨酸甲基化在先天抗病毒免疫中的基本功能。











































 京公网安备 11010802027423号
京公网安备 11010802027423号