Analytica Chimica Acta ( IF 6.2 ) Pub Date : 2021-09-21 , DOI: 10.1016/j.aca.2021.339084 Steffen Lippold 1 , Raashina Thavarajah 1 , Dietmar Reusch 2 , Manfred Wuhrer 1 , Simone Nicolardi 1
|
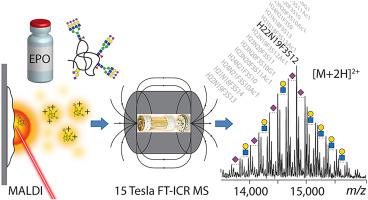
Recombinant human erythropoietin (EPO) is a complex therapeutic glycoprotein with three N- and one O-glycosylation sites. Glycosylation of EPO influences its safety and efficacy and is defined as a critical quality attribute. Thus, analytical methods for profiling EPO glycosylation are highly demanded. Owing to the complexity of the intact protein, information about EPO glycosylation is commonly derived from released glycan and glycopeptide analysis using mass spectrometry (MS). Alternatively, comprehensive insights into the glycoform heterogeneity of intact EPO are obtained using ESI MS-based methods with or without upfront separation of EPO glycoforms. MALDI MS, typically performed with TOF mass analyzers, has been also used for the analysis of intact EPO but, due to the poor glycoform resolution, has only provided limited glycoform information. Here, we present a MALDI FT-ICR MS method for the glycosylation profiling of intact EPO with improved glycoform resolution and without loss of sialic acid residues commonly observed in MALDI analysis. Three EPO variants were characterized in-depth and up to 199 glycoform compositions were assigned from the evaluation of doubly-charged ions, without any deconvolution of the mass spectra. Key glycosylation features such as sialylation, acetylation, and N-acetyllactosamine repeats were determined and found to agree with previously reported data obtained from orthogonal analyses. The developed method allowed for a fast and straightforward data acquisition and evaluation and can be potentially used for the high-throughput comparison of EPO samples throughout its manufacturing process.
中文翻译:

通过 MALDI FT-ICR 质谱法对完整促红细胞生成素进行糖型分析
重组人促红细胞生成素 (EPO) 是一种复杂的治疗性糖蛋白,具有三个N - 和一个O-糖基化位点。EPO 的糖基化影响其安全性和有效性,并被定义为关键的质量属性。因此,非常需要用于分析 EPO 糖基化的分析方法。由于完整蛋白质的复杂性,有关 EPO 糖基化的信息通常来自使用质谱 (MS) 进行的释放聚糖和糖肽分析。或者,使用基于 ESI MS 的方法(无论是否预先分离 EPO 糖型),都可以全面了解完整 EPO 的糖型异质性。MALDI MS 通常使用 TOF 质量分析器进行,也已用于分析完整的 EPO,但由于糖型分辨率较差,只能提供有限的糖型信息。这里,我们提出了一种 MALDI FT-ICR MS 方法,用于对完整 EPO 进行糖基化分析,具有更高的糖型分辨率,并且不会丢失 MALDI 分析中常见的唾液酸残基。对三种 EPO 变体进行了深入表征,通过对双电荷离子的评估确定了多达 199 种糖型组合物,而没有任何质谱解卷积。关键的糖基化特征,例如唾液酸化、乙酰化和确定了N-乙酰乳糖胺重复序列,发现与之前报道的从正交分析中获得的数据一致。开发的方法允许快速和直接的数据采集和评估,并有可能用于整个制造过程中 EPO 样品的高通量比较。



























 京公网安备 11010802027423号
京公网安备 11010802027423号