当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
De novo Assembly of the Benzenoid Ring as a Core Strategy for Synthesis of the Isoindolinone Natural Products Isohericerin, Erinacerin A, and Sterenin A
Organic Letters ( IF 4.9 ) Pub Date : 2021-09-20 , DOI: 10.1021/acs.orglett.1c02752 Chenlong Zhu 1 , Juntian Zhang 1 , Thomas R Hoye 1
Organic Letters ( IF 4.9 ) Pub Date : 2021-09-20 , DOI: 10.1021/acs.orglett.1c02752 Chenlong Zhu 1 , Juntian Zhang 1 , Thomas R Hoye 1
Affiliation
|
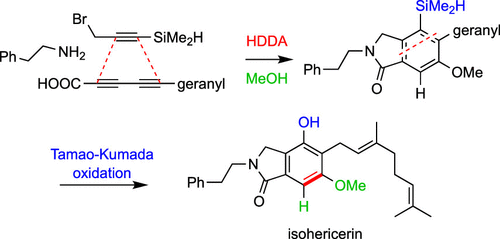
Here we describe the use of the hexadehydro-Diels–Alder (HDDA) reaction for the de novo construction of the isoindolinone scaffold and its application to the synthesis of the title natural products. The key isoindolinone-forming HDDA reaction involved an unprecedented substrate motif in which an amide carbonyl group was conjugated to the 4π 1,3-diyne component. In addition, a dimethylsilyl (−SiMe2H) substituent was exploited to trigger a Fleming–Tamao–Kumada oxidation for the installation of an essential phenolic hydroxyl group.
中文翻译:

苯环的从头组装作为异吲哚啉酮天然产物异蜡菌素、Erinacerin A 和 Sterenin A 合成的核心策略
在这里,我们描述了使用hexadehydro-Diels-Alder (HDDA) 反应从头构建异吲哚啉酮支架及其在标题天然产物合成中的应用。关键的异吲哚啉酮形成 HDDA 反应涉及前所未有的底物基序,其中酰胺羰基与 4π 1,3-二炔组分共轭。此外,利用二甲基甲硅烷基 (-SiMe 2 H) 取代基来触发 Fleming-Tamao-Kumada 氧化,以安装必需的酚羟基。
更新日期:2021-10-01
中文翻译:

苯环的从头组装作为异吲哚啉酮天然产物异蜡菌素、Erinacerin A 和 Sterenin A 合成的核心策略
在这里,我们描述了使用hexadehydro-Diels-Alder (HDDA) 反应从头构建异吲哚啉酮支架及其在标题天然产物合成中的应用。关键的异吲哚啉酮形成 HDDA 反应涉及前所未有的底物基序,其中酰胺羰基与 4π 1,3-二炔组分共轭。此外,利用二甲基甲硅烷基 (-SiMe 2 H) 取代基来触发 Fleming-Tamao-Kumada 氧化,以安装必需的酚羟基。











































 京公网安备 11010802027423号
京公网安备 11010802027423号