当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
One-Pot Construction of Indolo[2,3-b]quinoxalines through Ruthenium-Catalyzed Ortho C–H Bond Functionalization of 2-Arylquinoxalines with Sulfonyl Azides
Organic Letters ( IF 4.9 ) Pub Date : 2021-09-20 , DOI: 10.1021/acs.orglett.1c02837 Sudip Laru 1 , Suvam Bhattacharjee 1 , Sumit Ghosh 1 , Alakananda Hajra 1
Organic Letters ( IF 4.9 ) Pub Date : 2021-09-20 , DOI: 10.1021/acs.orglett.1c02837 Sudip Laru 1 , Suvam Bhattacharjee 1 , Sumit Ghosh 1 , Alakananda Hajra 1
Affiliation
|
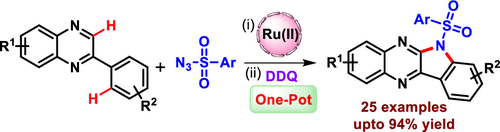
The synthesis of N-substituted indolo[2,3-b]quinoxalines has been developed through a Ru(II)-catalyzed ortho C–H functionalization of 2-arylquinoxalines with sulfonyl azides and further oxidation with 2,3-dichloro-5,6-dicyano-1,4-benzoquinone in one pot. This double C–N bond formation strategy provides a new efficient route for the preparation of a series of biologically relevant 6H-indolo[2,3-b]quinoxaline derivatives in up to 94% yield, suggesting a broad substrate scope applicability. The preliminary mechanistic studies reveal that the sequential C–N bond formations proceed through the formation of a five-membered ruthenacyclic intermediate in the first step and a radical mechanism in the second step.
中文翻译:

通过钌催化的 2-芳基喹喔啉与磺酰叠氮化物的邻位 C-H 键功能化一锅法构建吲哚 [2,3-b] 喹喔啉
N-取代的吲哚并[2,3- b ]喹喔啉的合成是通过Ru(II)催化的2-芳基喹喔啉与磺酰叠氮化物的邻位C-H官能化并用2,3-二氯-5进一步氧化而开发的, 6-二氰基-1,4-苯醌一锅。这种双C-N键形成策略为制备一系列生物相关的6 H-吲哚[2,3- b ]喹喔啉衍生物提供了一条新的有效途径,产率高达94%,表明底物适用范围广泛。初步的机理研究表明,连续的 C-N 键形成是通过第一步中五元钌环中间体的形成和第二步中自由基机制的形成进行的。
更新日期:2021-10-01
中文翻译:

通过钌催化的 2-芳基喹喔啉与磺酰叠氮化物的邻位 C-H 键功能化一锅法构建吲哚 [2,3-b] 喹喔啉
N-取代的吲哚并[2,3- b ]喹喔啉的合成是通过Ru(II)催化的2-芳基喹喔啉与磺酰叠氮化物的邻位C-H官能化并用2,3-二氯-5进一步氧化而开发的, 6-二氰基-1,4-苯醌一锅。这种双C-N键形成策略为制备一系列生物相关的6 H-吲哚[2,3- b ]喹喔啉衍生物提供了一条新的有效途径,产率高达94%,表明底物适用范围广泛。初步的机理研究表明,连续的 C-N 键形成是通过第一步中五元钌环中间体的形成和第二步中自由基机制的形成进行的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号