当前位置:
X-MOL 学术
›
Macromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tacticity Changes during Controlled Degradation of Polypropylene
Macromolecules ( IF 5.1 ) Pub Date : 2021-09-21 , DOI: 10.1021/acs.macromol.1c01383 Piet D. Iedema 1 , Klaas Remerie 2 , Desiree Seegers 3 , Kimberley B. McAuley 4
Macromolecules ( IF 5.1 ) Pub Date : 2021-09-21 , DOI: 10.1021/acs.macromol.1c01383 Piet D. Iedema 1 , Klaas Remerie 2 , Desiree Seegers 3 , Kimberley B. McAuley 4
Affiliation
|
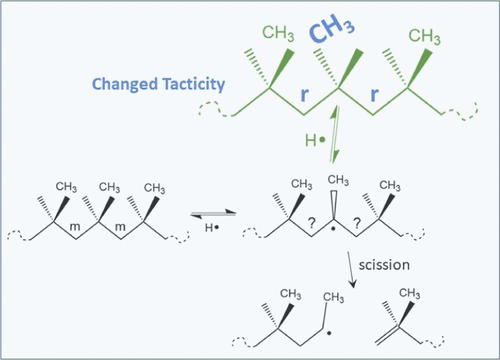
The controlled reactive degradation of polypropylene (CPP) in a twin-screw extruder has been investigated from the perspective of tacticity. CPP is initiated by the decomposition of a peroxide producing radicals that abstract hydrogen from the chain backbone creating tertiary radicals. Since C atoms with tertiary radicals have no longer sp3 configurations, they temporarily lose their stereospecificity. The C atoms resume stereospecificity upon termination, whereby methyl groups may assume a different orientation than before, causing a tacticity change. Degradation experiments in a twin-screw extruder of isotactic PP samples have been performed and tacticity changes are observed by C-13 NMR measurements revealing a decrease of isotactic mmmm pentads and increases of pentads with racemic sequence pairs. Statistical analysis of NMR data shows that the change of tacticity is nonrandom, i.e., units on the chain backbone are more likely to change orientation if they are close together. We attribute the nonrandomness to isomerization reactions, where through cyclic intermediates, a radical from one tertiary C carbon is transferred to another—radical chain walking. To validate this chain-walking hypothesis, a kinetic Monte Carlo simulation algorithm has been developed that predicts the change of the pentad distribution from the reactions taking place: initiation of tertiary radicals, transfer to polymer, chain walking, and termination by disproportionation. PP chains simulated consist of around 109 units with orientations of methyl groups inferred from the pentad distribution measured by NMR. Simulated pentad changes are compared to those measured from NMR, which reveal a relatively high racemic content. kMC simulations show that this can only be explained by assuming chain walking. Also, this isomerization via ring-shaped intermediates influences the patterns of changes in pentad distribution. These findings demonstrate that molecular weight changes are accompanied by tacticity changes. The kMC models confirmed by NMR data show that these tacticity changes are clustered locally along PP chains and that tacticity changes happen within the length scale of individual pentads. Chain-walking isomerization as a main cause of the tacticity changes is supported by predictions from the kMC model.
中文翻译:

聚丙烯受控降解过程中的规整变化
从立构规整的角度研究了双螺杆挤出机中聚丙烯 (CPP) 的受控反应降解。CPP 由过氧化物的分解引发,产生自由基,从链骨架中提取氢,产生叔自由基。由于具有叔自由基的 C 原子不再具有 sp 3构型,因此它们暂时失去了立体定向性。C 原子在终止时恢复立体定向性,由此甲基可能呈现与以前不同的取向,从而导致立构规整度发生变化。已经在双螺杆挤出机中进行了全同立构 PP 样品的降解实验,通过 C-13 NMR 测量观察到立构规整度的变化,显示全同立构mmmm五元组减少和五元组增加外消旋序列对。NMR 数据的统计分析表明,立构规整度的变化是非随机的,即如果链骨架上的单元靠得很近,它们更容易改变取向。我们将非随机性归因于异构化反应,其中通过环状中间体,来自一个叔 C 碳的自由基转移到另一个 - 自由基链行走。为了验证这种链游走假设,开发了一种动力学蒙特卡罗模拟算法,该算法可以预测发生的反应中五元组分布的变化:叔自由基的引发、转移到聚合物、链游走和歧化终止。模拟的 PP 链由大约 10 9从通过 NMR 测量的五单元组分布推断出具有甲基取向的单元。将模拟的五元组变化与 NMR 测量的变化进行比较,显示出相对较高的外消旋含量。kMC 模拟表明,这只能通过假设链式行走来解释。此外,这种通过环状中间体的异构化会影响五元组分布的变化模式。这些发现表明分子量的变化伴随着规整度的变化。核磁共振数据证实的 kMC 模型表明,这些规整度变化沿 PP 链局部聚集,并且规整度变化发生在单个五元组的长度尺度内。kMC 模型的预测支持链行走异构化作为策略变化的主要原因。
更新日期:2021-10-12
中文翻译:

聚丙烯受控降解过程中的规整变化
从立构规整的角度研究了双螺杆挤出机中聚丙烯 (CPP) 的受控反应降解。CPP 由过氧化物的分解引发,产生自由基,从链骨架中提取氢,产生叔自由基。由于具有叔自由基的 C 原子不再具有 sp 3构型,因此它们暂时失去了立体定向性。C 原子在终止时恢复立体定向性,由此甲基可能呈现与以前不同的取向,从而导致立构规整度发生变化。已经在双螺杆挤出机中进行了全同立构 PP 样品的降解实验,通过 C-13 NMR 测量观察到立构规整度的变化,显示全同立构mmmm五元组减少和五元组增加外消旋序列对。NMR 数据的统计分析表明,立构规整度的变化是非随机的,即如果链骨架上的单元靠得很近,它们更容易改变取向。我们将非随机性归因于异构化反应,其中通过环状中间体,来自一个叔 C 碳的自由基转移到另一个 - 自由基链行走。为了验证这种链游走假设,开发了一种动力学蒙特卡罗模拟算法,该算法可以预测发生的反应中五元组分布的变化:叔自由基的引发、转移到聚合物、链游走和歧化终止。模拟的 PP 链由大约 10 9从通过 NMR 测量的五单元组分布推断出具有甲基取向的单元。将模拟的五元组变化与 NMR 测量的变化进行比较,显示出相对较高的外消旋含量。kMC 模拟表明,这只能通过假设链式行走来解释。此外,这种通过环状中间体的异构化会影响五元组分布的变化模式。这些发现表明分子量的变化伴随着规整度的变化。核磁共振数据证实的 kMC 模型表明,这些规整度变化沿 PP 链局部聚集,并且规整度变化发生在单个五元组的长度尺度内。kMC 模型的预测支持链行走异构化作为策略变化的主要原因。











































 京公网安备 11010802027423号
京公网安备 11010802027423号