当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reversible electrochemical conversion from selenium to cuprous selenide
Chemical Communications ( IF 4.3 ) Pub Date : 2021-09-17 , DOI: 10.1039/d1cc03983k Sean K Sandstrom 1 , Heng Jiang 1 , Marcos Lucero 2 , Yunkai Xu 1 , Trenton C Gallagher 1 , Mengyuan Cao 1 , Zhenxing Feng 2 , Xiulei Ji 1
Chemical Communications ( IF 4.3 ) Pub Date : 2021-09-17 , DOI: 10.1039/d1cc03983k Sean K Sandstrom 1 , Heng Jiang 1 , Marcos Lucero 2 , Yunkai Xu 1 , Trenton C Gallagher 1 , Mengyuan Cao 1 , Zhenxing Feng 2 , Xiulei Ji 1
Affiliation
|
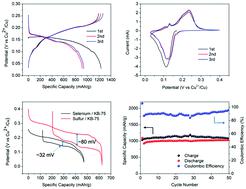
Using elemental selenium as an electrode, the redox-active Cu2+/Cu+ ion is reversibly hosted via the sequential conversion reactions of Se → CuSe → Cu3Se2 → Cu2Se. The four-electron redox process from Se to Cu2Se produces a high initial specific capacity of 1233 mA h g−1 based on the mass of selenium alone or 472 mA h g−1 based on the mass of Cu2Se, the fully discharged product.
中文翻译:

从硒到硒化亚铜的可逆电化学转化
使用元素硒作为电极,氧化还原活性 Cu 2+ /Cu +离子通过Se → CuSe → Cu 3 Se 2 → Cu 2 Se的顺序转化反应可逆地承载。从 Se 到 Cu 2 Se的四电子氧化还原过程产生了 1233 mA hg -1的高初始比容量,基于单独的硒质量或 472 mA hg -1基于完全放电产物Cu 2 Se的质量.
更新日期:2021-09-21
中文翻译:

从硒到硒化亚铜的可逆电化学转化
使用元素硒作为电极,氧化还原活性 Cu 2+ /Cu +离子通过Se → CuSe → Cu 3 Se 2 → Cu 2 Se的顺序转化反应可逆地承载。从 Se 到 Cu 2 Se的四电子氧化还原过程产生了 1233 mA hg -1的高初始比容量,基于单独的硒质量或 472 mA hg -1基于完全放电产物Cu 2 Se的质量.











































 京公网安备 11010802027423号
京公网安备 11010802027423号