当前位置:
X-MOL 学术
›
ChemBioChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Protein Design with Fluoroprolines: 4,4-Difluoroproline Does Not Eliminate the Rate-Limiting Step of Thioredoxin Folding
ChemBioChem ( IF 2.6 ) Pub Date : 2021-09-21 , DOI: 10.1002/cbic.202100418 Jennie O' Loughlin 1 , Silvia Napolitano 2 , Marina Rubini 1
ChemBioChem ( IF 2.6 ) Pub Date : 2021-09-21 , DOI: 10.1002/cbic.202100418 Jennie O' Loughlin 1 , Silvia Napolitano 2 , Marina Rubini 1
Affiliation
|
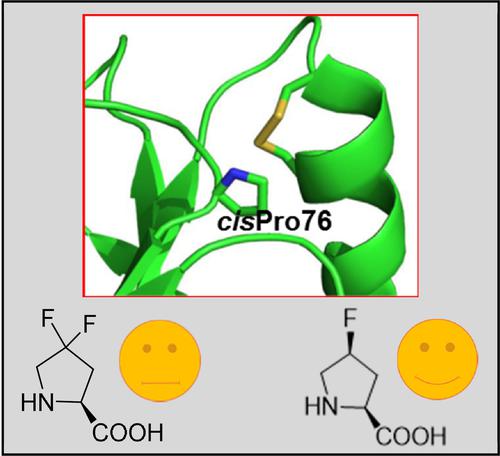
The incorporation of 4,4-difluoroproline into the conserved cis prolyl-peptide bond of the E. coli thioredoxin variant Trx1P neither eliminated the bottleneck of the thioredoxin refolding reaction nor had an impact on the attainment of the cis/trans equilibrium in the unfolded state. Our results suggest that the cis/trans isomerisation rates predicted for fluorinated proline analogues in the context of short model peptides are not applicable in the context of tertiary structures.

中文翻译:

用氟脯氨酸设计蛋白质:4,4-二氟脯氨酸不会消除硫氧还蛋白折叠的限速步骤
将4,4-二氟脯氨酸掺入大肠杆菌硫氧还蛋白变体 Trx1P 的保守顺式脯氨酰肽键中,既没有消除硫氧还蛋白重折叠反应的瓶颈,也没有影响在展开状态下实现顺式/反式平衡. 我们的结果表明,在短模型肽的背景下预测的氟化脯氨酸类似物的顺式/反式异构化速率不适用于三级结构的背景下。

更新日期:2021-09-21

中文翻译:

用氟脯氨酸设计蛋白质:4,4-二氟脯氨酸不会消除硫氧还蛋白折叠的限速步骤
将4,4-二氟脯氨酸掺入大肠杆菌硫氧还蛋白变体 Trx1P 的保守顺式脯氨酰肽键中,既没有消除硫氧还蛋白重折叠反应的瓶颈,也没有影响在展开状态下实现顺式/反式平衡. 我们的结果表明,在短模型肽的背景下预测的氟化脯氨酸类似物的顺式/反式异构化速率不适用于三级结构的背景下。











































 京公网安备 11010802027423号
京公网安备 11010802027423号