Joule ( IF 38.6 ) Pub Date : 2021-09-21 , DOI: 10.1016/j.joule.2021.08.013 Rui Kai Miao 1 , Yi Xu 1 , Adnan Ozden 1 , Anthony Robb 1 , Colin P. O’Brien 1 , Christine M. Gabardo 1 , Geonhui Lee 2 , Jonathan P. Edwards 1 , Jianan Erick Huang 2 , Mengyang Fan 1 , Xue Wang 2 , Shijie Liu 1 , Yu Yan 2 , Edward H. Sargent 2 , David Sinton 1
|
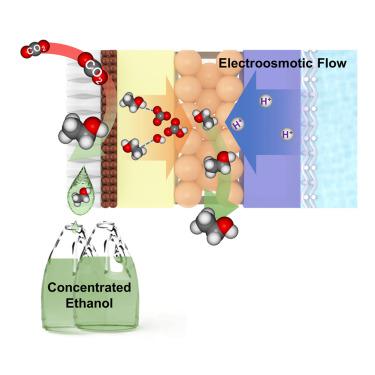
Electrochemical reduction of carbon dioxide (CO2RR) converts intermittent renewable energy into high energy density fuels, such as ethanol. Membrane electrode assembly (MEA) electrolyzers are particularly well-suited for CO2-to-ethanol conversion in view of their low ohmic resistance and high stability. However, over 75% of the ethanol produced at the cathode migrates through the membrane where it is diluted by the anolyte and may be oxidized. The ethanol concentration that results is two orders of magnitude below the 10 wt % standard set by the incumbent industrial process, fermentation. Here, we reverse the direction of ion and electroosmotic transport by means of a porous proton exchange layer, thereby blocking both the convective and diffusive routes of ethanol loss. With this strategy, we eliminate ethanol crossover to the anode (< 1%) and achieve an ethanol concentration of 13.1 wt % directly from the cathode outlet.
中文翻译:

电渗流控制中性产品,并能够从 CO2 中电解生产浓缩乙醇
二氧化碳的电化学还原 (CO 2 RR) 将间歇性可再生能源转化为高能量密度燃料,例如乙醇。膜电极组件 (MEA) 电解槽特别适用于 CO 2鉴于它们的低欧姆电阻和高稳定性,可以将其转化为乙醇。然而,在阴极产生的超过 75% 的乙醇通过膜迁移,在那里它被阳极电解液稀释并可能被氧化。产生的乙醇浓度比现有工业过程发酵设定的 10 wt% 标准低两个数量级。在这里,我们通过多孔质子交换层反转离子和电渗传输的方向,从而阻止乙醇损失的对流和扩散途径。通过这种策略,我们消除了乙醇向阳极的交叉(< 1%),并直接从阴极出口实现了 13.1 wt% 的乙醇浓度。











































 京公网安备 11010802027423号
京公网安备 11010802027423号