International Journal of Hydrogen Energy ( IF 7.2 ) Pub Date : 2021-09-21 , DOI: 10.1016/j.ijhydene.2021.09.056 Na Ye 1 , Zhao Jiang 1 , Tao Fang 1
|
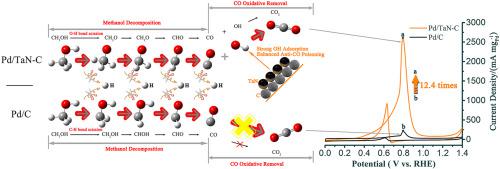
The low-palladium Pd/TaN–C catalyst is synthesized by a surfactant-free solvothermal approach and exhibits high activity (2613.18 mA mgPd−1), durability and CO tolerance for MOR (methanol oxidation reaction) in alkaline media, 12.4 folds that of the commercial Pd/C. XPS and electrochemical results indicate that the interfacial Pd–TaNO bond is generated. This also brings the enhancement of OHad adsorption responsible for anti-CO poisoning ability. Density Functional Theory (DFT) calculations indicate that the reaction pathway and the rate-determining step are changed for methanol decomposition to CO on the Pd4/TaN(001) surface compared with Pd (111). The preferred pathway can be described as: CH3OH→CH3O→CH2O→CHO→CO. Furthermore, the results indicate that the adsorption of OH is enhanced and the energy barrier of COOH formation from CO + OH is reduced with the high concentration of hydroxyl on the Pd4/TaN(001) surface, further confirming the bi-functional effect of hydroxyl on the CO tolerance.
中文翻译:

探讨通过促进 CO 耐受性对用 TaN 改性的 Pd 催化剂增强甲醇电氧化机制:结合实验和理论研究
低钯 Pd/TaN-C 催化剂是通过无表面活性剂的溶剂热法合成的,在碱性介质中对 MOR(甲醇氧化反应)表现出高活性(2613.18 mA mg Pd -1)、耐久性和 CO 耐受性,是其 12.4 倍商业 Pd/C。XPS 和电化学结果表明生成了界面 Pd-TaNO 键。这也带来了负责抗 CO 中毒能力的 OH ad吸附的增强。密度泛函理论 (DFT) 计算表明,与 Pd (111) 相比,Pd 4 /TaN(001) 表面上甲醇分解为 CO 的反应途径和速率决定步骤发生了变化。优选途径可描述为:CH 3 OH→CH 3O→CH 2 O→CHO→CO。此外,结果表明,随着 Pd 4 /TaN(001) 表面上高浓度的羟基,OH 的吸附增强,CO + OH 形成 COOH 的能垒降低,进一步证实了双功能效应羟基对 CO 耐受性的影响。



























 京公网安备 11010802027423号
京公网安备 11010802027423号