当前位置:
X-MOL 学术
›
J. Mater. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Microenvironment-responsive DNA-conjugated albumin nanocarriers for targeted therapy
Journal of Materials Chemistry B ( IF 6.1 ) Pub Date : 2021-08-31 , DOI: 10.1039/d1tb01022k Jiayu Yu 1, 2 , Jianing Zhang 1, 2 , Jing Jin 1 , Wei Jiang 1, 2
Journal of Materials Chemistry B ( IF 6.1 ) Pub Date : 2021-08-31 , DOI: 10.1039/d1tb01022k Jiayu Yu 1, 2 , Jianing Zhang 1, 2 , Jing Jin 1 , Wei Jiang 1, 2
Affiliation
|
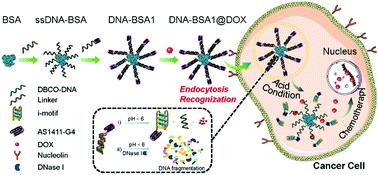
Drug delivery with accurate targeting and efficient treatment has become an essential strategy for cancer therapy. Two nanocarriers based on bovine serum albumin (BSA) and DNA were synthesized via click chemistry and DNA hybridization reactions (DNA–BSA1 and DNA–BSA2). One of the hybridized oligonucleotides, Linker1, in DNA–BSA1 included a pH-sensitive i-motif sequence and a cancer cell-targeted guanine-quadruplex-structured AS1411 aptamer sequence, and the other, Linker2, in DNA–BSA2 had only the same pH-sensitive i-motif sequence. Doxorubicin (DOX) molecules could be quickly and preferentially intercalated into double-stranded DNA via non-covalent interactions, and the encapsulation efficiency of DNA–BSA1 and DNA–BSA2 was almost 100% and 87.5%, respectively. As a mimic of the cancer cell microenvironment, a pH-trigger and a deoxyribonuclease I (DNase I)-trigger release mechanism was individually proposed to explain the dynamic release of the DNA–BSA@DOX under acidic conditions and the presence of DNase I in vitro. Intracellular uptake and cytotoxicity experiments confirmed that the nanocarrier DNA–BSA1@DOX had accurate targeting and efficient treatment towards cancer cells due to the high affinity and specificity of AS1411 to nucleolin, which is overexpressed in cancer cells. Furthermore, in vivo studies showed that the nanocarrier system could efficiently inhibit tumor growth. Therefore, the entire bio-based nanocarrier DNA–BSA is a promising candidate for the loading and release of anti-cancer drugs for accurate delivery and efficient treatment.
中文翻译:

用于靶向治疗的微环境响应性 DNA 偶联白蛋白纳米载体
精确靶向和有效治疗的药物输送已成为癌症治疗的重要策略。通过点击化学和 DNA 杂交反应(DNA-BSA1 和 DNA-BSA2)合成了两种基于牛血清白蛋白 (BSA) 和 DNA 的纳米载体。DNA-BSA1 中的一个杂交寡核苷酸 Linker1 包括一个 pH 敏感 i-motif 序列和一个癌细胞靶向的鸟嘌呤四链体结构的 AS1411 适体序列,而 DNA-BSA2 中的另一个 Linker2 仅具有相同的pH 敏感的 i 基序序列。多柔比星 (DOX) 分子可以通过非共价相互作用,DNA-BSA1 和 DNA-BSA2 的包封率分别接近 100% 和 87.5%。作为癌细胞微环境的模拟物,分别提出了 pH 触发和脱氧核糖核酸酶 I (DNase I) 触发释放机制来解释 DNA-BSA@DOX 在酸性条件下的动态释放和 DNase I 的存在。体外。细胞内摄取和细胞毒性实验证实,由于 AS1411 对在癌细胞中过表达的核仁素具有高亲和力和特异性,因此纳米载体 DNA-BSA1@DOX 对癌细胞具有准确的靶向和有效的治疗作用。此外,在体内研究表明,纳米载体系统可以有效地抑制肿瘤生长。因此,整个生物基纳米载体 DNA-BSA 是一种很有前途的候选者,可用于装载和释放抗癌药物,以实现准确递送和高效治疗。
更新日期:2021-09-20
中文翻译:

用于靶向治疗的微环境响应性 DNA 偶联白蛋白纳米载体
精确靶向和有效治疗的药物输送已成为癌症治疗的重要策略。通过点击化学和 DNA 杂交反应(DNA-BSA1 和 DNA-BSA2)合成了两种基于牛血清白蛋白 (BSA) 和 DNA 的纳米载体。DNA-BSA1 中的一个杂交寡核苷酸 Linker1 包括一个 pH 敏感 i-motif 序列和一个癌细胞靶向的鸟嘌呤四链体结构的 AS1411 适体序列,而 DNA-BSA2 中的另一个 Linker2 仅具有相同的pH 敏感的 i 基序序列。多柔比星 (DOX) 分子可以通过非共价相互作用,DNA-BSA1 和 DNA-BSA2 的包封率分别接近 100% 和 87.5%。作为癌细胞微环境的模拟物,分别提出了 pH 触发和脱氧核糖核酸酶 I (DNase I) 触发释放机制来解释 DNA-BSA@DOX 在酸性条件下的动态释放和 DNase I 的存在。体外。细胞内摄取和细胞毒性实验证实,由于 AS1411 对在癌细胞中过表达的核仁素具有高亲和力和特异性,因此纳米载体 DNA-BSA1@DOX 对癌细胞具有准确的靶向和有效的治疗作用。此外,在体内研究表明,纳米载体系统可以有效地抑制肿瘤生长。因此,整个生物基纳米载体 DNA-BSA 是一种很有前途的候选者,可用于装载和释放抗癌药物,以实现准确递送和高效治疗。











































 京公网安备 11010802027423号
京公网安备 11010802027423号