当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Necessity of Flanking Repeats R1′ and R8′ of Human Pumilio1 Protein for RNA Binding
Biochemistry ( IF 2.9 ) Pub Date : 2021-09-19 , DOI: 10.1021/acs.biochem.1c00445 Kento Nakamura 1 , Taishu Nakao 1 , Tomoaki Mori 1 , Serika Ohno 1 , Yusuke Fujita 1 , Keisuke Masaoka 1 , Kazuki Sakabayashi 1 , Koichi Mori 1 , Takamasa Tobimatsu 1 , Takashi Sera 1
Biochemistry ( IF 2.9 ) Pub Date : 2021-09-19 , DOI: 10.1021/acs.biochem.1c00445 Kento Nakamura 1 , Taishu Nakao 1 , Tomoaki Mori 1 , Serika Ohno 1 , Yusuke Fujita 1 , Keisuke Masaoka 1 , Kazuki Sakabayashi 1 , Koichi Mori 1 , Takamasa Tobimatsu 1 , Takashi Sera 1
Affiliation
|
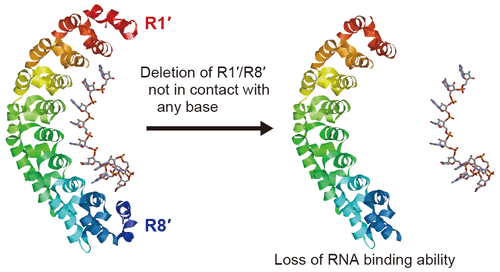
Human Pumilio (hPUM) is a structurally well-analyzed RNA-binding protein that has been used recently for artificial RNA binding. Structural analysis revealed that amino acids at positions 12, 13, and 16 in the repeats from R1 to R8 each contact one specific RNA base in the eight-nucleotide RNA target. The functions of the N- and C-terminal flanking repeats R1′ and R8′, however, remain unclear. Here, we report how the repeats contribute to overall RNA binding. We first prepared three mutants in which R1′ and/or R8′ were deleted and then analyzed RNA binding using gel shift assays. The assays showed that all deletion mutants bound to their target less than the original hPUM, but that R1′ contributed more than R8′, unlike Drosophila PUM. We next investigated which amino acid residues of R1′ or R8′ were responsible for RNA binding. With detailed analysis of the protein tertiary structure, we found a hydrophobic core in each of the repeats. We therefore mutated all hydrophobic amino residues in each core to alanine. The gel shift assays with the resulting mutants revealed that both hydrophobic cores contributed to the RNA binding: especially the hydrophobic core of R1′ had a significant influence. In the present study, we demonstrated that the flanking R1′ and R8′ repeats are indispensable for RNA binding of hPUM and suggest that hydrophobic R1′–R1 interactions may stabilize the whole hPUM structure.
中文翻译:

人 Pumilio1 蛋白侧翼重复 R1' 和 R8' 用于 RNA 结合的必要性
Human Pumilio (hPUM) 是一种结构分析良好的 RNA 结合蛋白,最近已用于人工 RNA 结合。结构分析表明,在从 R1 到 R8 的重复序列中,第 12、13 和 16 位的氨基酸分别与八核苷酸 RNA 靶标中的一个特定 RNA 碱基接触。然而,N 端和 C 端侧翼重复序列 R1' 和 R8' 的功能仍不清楚。在这里,我们报告重复如何有助于整体 RNA 结合。我们首先制备了三个突变体,其中 R1' 和/或 R8' 被删除,然后使用凝胶位移测定分析 RNA 结合。分析表明,所有缺失突变体与其靶标的结合比原始 hPUM 少,但与果蝇不同,R1' 贡献大于 R8'泵。我们接下来研究了 R1' 或 R8' 的哪些氨基酸残基负责 RNA 结合。通过对蛋白质三级结构的详细分析,我们在每个重复序列中发现了一个疏水核心。因此,我们将每个核心中的所有疏水性氨基残基突变为丙氨酸。对所得突变体的凝胶位移分析表明,两个疏水核心都有助于 RNA 结合:尤其是 R1' 的疏水核心具有显着影响。在本研究中,我们证明了侧翼 R1' 和 R8' 重复序列对于 hPUM 的 RNA 结合是必不可少的,并表明疏水性 R1'-R1 相互作用可以稳定整个 hPUM 结构。
更新日期:2021-10-12
中文翻译:

人 Pumilio1 蛋白侧翼重复 R1' 和 R8' 用于 RNA 结合的必要性
Human Pumilio (hPUM) 是一种结构分析良好的 RNA 结合蛋白,最近已用于人工 RNA 结合。结构分析表明,在从 R1 到 R8 的重复序列中,第 12、13 和 16 位的氨基酸分别与八核苷酸 RNA 靶标中的一个特定 RNA 碱基接触。然而,N 端和 C 端侧翼重复序列 R1' 和 R8' 的功能仍不清楚。在这里,我们报告重复如何有助于整体 RNA 结合。我们首先制备了三个突变体,其中 R1' 和/或 R8' 被删除,然后使用凝胶位移测定分析 RNA 结合。分析表明,所有缺失突变体与其靶标的结合比原始 hPUM 少,但与果蝇不同,R1' 贡献大于 R8'泵。我们接下来研究了 R1' 或 R8' 的哪些氨基酸残基负责 RNA 结合。通过对蛋白质三级结构的详细分析,我们在每个重复序列中发现了一个疏水核心。因此,我们将每个核心中的所有疏水性氨基残基突变为丙氨酸。对所得突变体的凝胶位移分析表明,两个疏水核心都有助于 RNA 结合:尤其是 R1' 的疏水核心具有显着影响。在本研究中,我们证明了侧翼 R1' 和 R8' 重复序列对于 hPUM 的 RNA 结合是必不可少的,并表明疏水性 R1'-R1 相互作用可以稳定整个 hPUM 结构。











































 京公网安备 11010802027423号
京公网安备 11010802027423号