当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
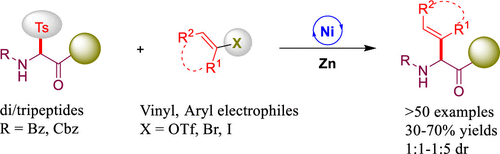
Alkenylation and Arylation of Peptides via Ni-Catalyzed Reductive Coupling of α-C-Tosyl Peptides with Csp2 Triflates/Halides
Organic Letters ( IF 5.2 ) Pub Date : 2021-09-20 , DOI: 10.1021/acs.orglett.1c02601 Xianghua Tao 1 , Guobin Ma 1 , Yanhong Song 1 , Yunrong Chen 2 , Qun Qian 1 , Deli Sun 1 , Hegui Gong 1
Organic Letters ( IF 5.2 ) Pub Date : 2021-09-20 , DOI: 10.1021/acs.orglett.1c02601 Xianghua Tao 1 , Guobin Ma 1 , Yanhong Song 1 , Yunrong Chen 2 , Qun Qian 1 , Deli Sun 1 , Hegui Gong 1
Affiliation
|
A Ni-catalyzed reductive cross-coupling between α-C-tosyl peptides and Csp2 triflates/halides has been developed. This protocol enables the formation of various unnatural di- and tripeptides containing vinyl and aryl side chains, and it expands the applications of Ni-catalyzed reductive cross-coupling in late-stage diversification of peptides.
中文翻译:

通过 α-C-甲苯磺酰肽与 Csp2 三氟甲磺酸盐/卤化物的镍催化还原偶联使肽的烯基化和芳基化
已经开发了α-C-甲苯磺酰肽和 Csp 2三氟甲磺酸盐/卤化物之间的 Ni 催化还原交叉偶联。该协议能够形成各种含有乙烯基和芳基侧链的非天然二肽和三肽,并扩展了镍催化还原交叉偶联在肽后期多样化中的应用。
更新日期:2021-10-01
中文翻译:

通过 α-C-甲苯磺酰肽与 Csp2 三氟甲磺酸盐/卤化物的镍催化还原偶联使肽的烯基化和芳基化
已经开发了α-C-甲苯磺酰肽和 Csp 2三氟甲磺酸盐/卤化物之间的 Ni 催化还原交叉偶联。该协议能够形成各种含有乙烯基和芳基侧链的非天然二肽和三肽,并扩展了镍催化还原交叉偶联在肽后期多样化中的应用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号