Journal of Environmental Chemical Engineering ( IF 7.4 ) Pub Date : 2021-09-20 , DOI: 10.1016/j.jece.2021.106382 Lázaro Adrián González-Fernández 1 , Nahum Andrés Medellín-Castillo 1 , Raúl Ocampo-Pérez 2 , Héctor Hernández-Mendoza 3 , María Selene Berber-Mendoza 1 , Cristóbal Aldama-Aguilera 1
|
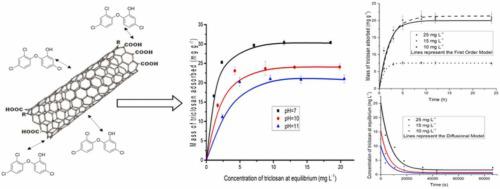
In this work, Simple Walled Carbon Nanotubes (SWCNTs) were used as adsorbents to remove triclosan (TCS) from aqueous solution. The material characterization consisted of SEM/EDS and TEM/EDS analysis, surface characterization, and the determination of the pH at the point of zero charge. The effect of solution pH on the adsorption equilibrium was investigated in batch experiments. The overall adsorption rate of TCS on SWCNTs was investigated by applying kinetic models and a diffusional model based on pore volume diffusion. The results showed that a maximum adsorption capacity of TCS onto SWCNTs is obtained at a pH value of 7, which is 30.3 mg g−1. The adsorption kinetics of TCS on SWCNTs was interpreted correctly by the First Order Kinetic Model and the Diffusional Model. The values of Dep varied from 1.06 to 1.20 × 10−8 cm2 s−1 and increased discretely as the mass of TCS adsorbed at equilibrium increased.
中文翻译:

三氯生在单壁碳纳米管上的吸附平衡和动力学模型
在这项工作中,简单壁碳纳米管 (SWCNT) 用作吸附剂以从水溶液中去除三氯生 (TCS)。材料表征包括 SEM/EDS 和 TEM/EDS 分析、表面表征和零电荷点 pH 值的测定。在批量实验中研究了溶液 pH 值对吸附平衡的影响。通过应用动力学模型和基于孔体积扩散的扩散模型,研究了 TCS 在 SWCNT 上的整体吸附率。结果表明,在 pH 值为 7 时,TCS 对 SWCNT 的最大吸附容量为 30.3 mg g -1. TCS 在 SWCNT 上的吸附动力学被一阶动力学模型和扩散模型正确解释。Dep 的值在1.06 到1.20 × 10 -8 cm 2 s -1 之间变化,并且随着平衡时吸附的TCS 质量的增加而离散地增加。











































 京公网安备 11010802027423号
京公网安备 11010802027423号