当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Characterization of Americium and Curium Complexes with the Protein Lanmodulin: A Potential Macromolecular Mechanism for Actinide Mobility in the Environment
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-09-20 , DOI: 10.1021/jacs.1c07103 Gauthier J-P Deblonde 1, 2 , Joseph A Mattocks 3 , Huan Wang 4 , Eric M Gale 4 , Annie B Kersting 1, 2 , Mavrik Zavarin 1, 2 , Joseph A Cotruvo 3
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-09-20 , DOI: 10.1021/jacs.1c07103 Gauthier J-P Deblonde 1, 2 , Joseph A Mattocks 3 , Huan Wang 4 , Eric M Gale 4 , Annie B Kersting 1, 2 , Mavrik Zavarin 1, 2 , Joseph A Cotruvo 3
Affiliation
|
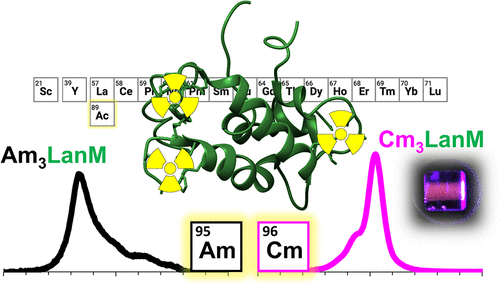
Anthropogenic radionuclides, including long-lived heavy actinides such as americium and curium, represent the primary long-term challenge for management of nuclear waste. The potential release of these wastes into the environment necessitates understanding their interactions with biogeochemical compounds present in nature. Here, we characterize the interactions between the heavy actinides, Am3+ and Cm3+, and the natural lanthanide-binding protein, lanmodulin (LanM). LanM is produced abundantly by methylotrophic bacteria, including Methylorubrum extorquens, that are widespread in the environment. We determine the first stability constant for an Am3+-protein complex (Am3LanM) and confirm the results with Cm3LanM, indicating a ∼5-fold higher affinity than that for lanthanides with most similar ionic radius, Nd3+ and Sm3+, and making LanM the strongest known heavy actinide-binding protein. The protein’s high selectivity over 243Am’s daughter nuclide 239Np enables lab-scale actinide-actinide separations as well as provides insight into potential protein-driven mobilization for these actinides in the environment. The luminescence properties of the Cm3+-LanM complex, and NMR studies of Gd3+-LanM, reveal that lanmodulin-bound f-elements possess two coordinated solvent molecules across a range of metal ionic radii. Finally, we show under a wide range of environmentally relevant conditions that lanmodulin effectively outcompetes desferrioxamine B, a hydroxamate siderophore previously proposed to be important in trivalent actinide mobility. These results suggest that natural lanthanide-binding proteins such as lanmodulin may play important roles in speciation and mobility of actinides in the environment; it also suggests that protein-based biotechnologies may provide a new frontier in actinide remediation, detection, and separations.
中文翻译:

镅和锔配合物与蛋白质 Lanmodulin 的表征:锕系元素在环境中迁移的潜在大分子机制
人为放射性核素,包括长寿命的重锕系元素,如镅和锔,是核废料管理面临的主要长期挑战。这些废物可能会释放到环境中,因此需要了解它们与自然界中存在的生物地球化学化合物的相互作用。在这里,我们描述了重锕系元素 Am 3+和 Cm 3+与天然镧系元素结合蛋白 lanmodulin (LanM)之间的相互作用。LanM 由甲基营养菌大量产生,包括在环境中广泛存在的Methylorubrum extorquens 。我们确定了 Am 3+蛋白复合物 (Am 3 LanM)的第一个稳定常数,并用 Cm 确认了结果3 LanM,表明与具有最相似离子半径的镧系元素 Nd 3+和 Sm 3+相比,亲和力高约 5 倍,并使 LanM 成为已知最强的重锕系元素结合蛋白。该蛋白质对243 Am 的子核素239 Np 的高选择性使实验室规模的锕系元素-锕系元素分离成为可能,并提供了对这些锕系元素在环境中潜在的蛋白质驱动动员的洞察力。Cm 3+ -LanM配合物的发光特性和Gd 3+的核磁共振研究-LanM,表明与 lanmodulin 结合的 f 元素在一系列金属离子半径范围内拥有两个协调的溶剂分子。最后,我们表明在广泛的环境相关条件下,lanmodulin 有效地胜过去铁胺 B,去铁胺 B 是一种先前被认为对三价锕系元素流动性很重要的异羟肟酸铁载体。这些结果表明,天然镧系元素结合蛋白(如 lanmodulin)可能在锕系元素在环境中的物种形成和迁移中发挥重要作用;它还表明,基于蛋白质的生物技术可能为锕系元素的修复、检测和分离开辟新的领域。
更新日期:2021-09-29
中文翻译:

镅和锔配合物与蛋白质 Lanmodulin 的表征:锕系元素在环境中迁移的潜在大分子机制
人为放射性核素,包括长寿命的重锕系元素,如镅和锔,是核废料管理面临的主要长期挑战。这些废物可能会释放到环境中,因此需要了解它们与自然界中存在的生物地球化学化合物的相互作用。在这里,我们描述了重锕系元素 Am 3+和 Cm 3+与天然镧系元素结合蛋白 lanmodulin (LanM)之间的相互作用。LanM 由甲基营养菌大量产生,包括在环境中广泛存在的Methylorubrum extorquens 。我们确定了 Am 3+蛋白复合物 (Am 3 LanM)的第一个稳定常数,并用 Cm 确认了结果3 LanM,表明与具有最相似离子半径的镧系元素 Nd 3+和 Sm 3+相比,亲和力高约 5 倍,并使 LanM 成为已知最强的重锕系元素结合蛋白。该蛋白质对243 Am 的子核素239 Np 的高选择性使实验室规模的锕系元素-锕系元素分离成为可能,并提供了对这些锕系元素在环境中潜在的蛋白质驱动动员的洞察力。Cm 3+ -LanM配合物的发光特性和Gd 3+的核磁共振研究-LanM,表明与 lanmodulin 结合的 f 元素在一系列金属离子半径范围内拥有两个协调的溶剂分子。最后,我们表明在广泛的环境相关条件下,lanmodulin 有效地胜过去铁胺 B,去铁胺 B 是一种先前被认为对三价锕系元素流动性很重要的异羟肟酸铁载体。这些结果表明,天然镧系元素结合蛋白(如 lanmodulin)可能在锕系元素在环境中的物种形成和迁移中发挥重要作用;它还表明,基于蛋白质的生物技术可能为锕系元素的修复、检测和分离开辟新的领域。











































 京公网安备 11010802027423号
京公网安备 11010802027423号