当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ni/Photoredox-Catalyzed Enantioselective Cross-Electrophile Coupling of Styrene Oxides with Aryl Iodides
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-09-20 , DOI: 10.1021/jacs.1c08105 Sii Hong Lau 1, 2 , Meredith A Borden 1 , Talia J Steiman 1 , Lucy S Wang 1 , Marvin Parasram 1 , Abigail G Doyle 1, 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-09-20 , DOI: 10.1021/jacs.1c08105 Sii Hong Lau 1, 2 , Meredith A Borden 1 , Talia J Steiman 1 , Lucy S Wang 1 , Marvin Parasram 1 , Abigail G Doyle 1, 2
Affiliation
|
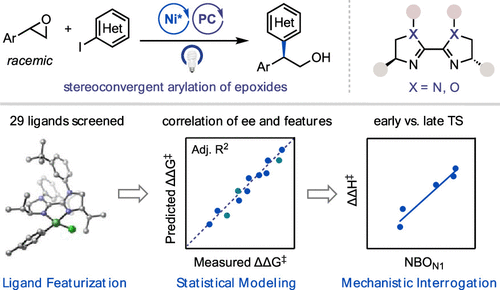
A Ni/photoredox-catalyzed enantioselective reductive coupling of styrene oxides and aryl iodides is reported. This reaction affords access to enantioenriched 2,2-diarylalcohols from racemic epoxides via a stereoconvergent mechanism. Multivariate linear regression (MVLR) analysis with 29 bioxazoline (BiOx) and biimidazoline (BiIm) ligands revealed that enantioselectivity correlates with electronic properties of the ligands, with more electron-donating ligands affording higher ee’s. Experimental and computational mechanistic studies were conducted, lending support to the hypothesis that reductive elimination is enantiodetermining and the electronic character of the ligands influences the enantioselectivity by altering the position of the transition state structure along the reaction coordinate. This study demonstrates the benefits of utilizing statistical modeling as a platform for mechanistic understanding and provides new insight into an emerging class of chiral ligands for stereoconvergent Ni and Ni/photoredox cross-coupling.
中文翻译:

Ni/光氧化还原催化的苯乙烯氧化物与芳基碘化物的对映选择性交叉电偶联
报道了一种 Ni/光氧化还原催化的氧化苯乙烯和芳基碘化物的对映选择性还原偶联。该反应通过立体收敛机制从外消旋环氧化物中获得对映体富集的 2,2-二芳基醇。对 29 种生物恶唑啉 (BiOx) 和联咪唑啉 (BiIm) 配体的多元线性回归 (MVLR) 分析表明,对映选择性与配体的电子特性相关,更多的供电子配体提供更高的 ee。进行了实验和计算机理研究,支持还原消除是对映决定的假设,并且配体的电子特性通过改变过渡态结构沿反应坐标的位置来影响对映选择性。
更新日期:2021-09-29
中文翻译:

Ni/光氧化还原催化的苯乙烯氧化物与芳基碘化物的对映选择性交叉电偶联
报道了一种 Ni/光氧化还原催化的氧化苯乙烯和芳基碘化物的对映选择性还原偶联。该反应通过立体收敛机制从外消旋环氧化物中获得对映体富集的 2,2-二芳基醇。对 29 种生物恶唑啉 (BiOx) 和联咪唑啉 (BiIm) 配体的多元线性回归 (MVLR) 分析表明,对映选择性与配体的电子特性相关,更多的供电子配体提供更高的 ee。进行了实验和计算机理研究,支持还原消除是对映决定的假设,并且配体的电子特性通过改变过渡态结构沿反应坐标的位置来影响对映选择性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号