Redox Biology ( IF 10.7 ) Pub Date : 2021-09-20 , DOI: 10.1016/j.redox.2021.102141 Naijin Zhang 1 , Ying Zhang 1 , Boquan Wu 2 , Shaojun Wu 2 , Shilong You 2 , Saien Lu 2 , Jingwei Liu 3 , Xinyue Huang 2 , Jiaqi Xu 2 , Liu Cao 3 , Yingxian Sun 1
|
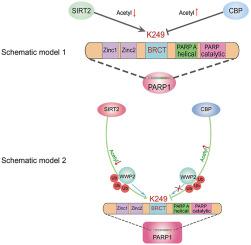
Poly(ADP-ribose) polymerase 1 (PARP1) has a major regulatory role in cardiovascular disease. However, inhibiting PARP1 activity does not significantly improve clinical outcomes of cardiovascular disease, which suggests that the regulatory mechanism of PARP1 in cardiovascular disease is unclear. Here, we focused on deacetylation regulatory mechanisms of PARP1 and crosstalk of PARP1 post-translational modifications. We uncovered the crucial molecular interactions and protein modifications of deacetylase Sirtuin 2 (SIRT2) and PARP1 in vascular damage. The results showed that SIRT2 was involved in this process and oxidative stress damage factor PARP1 was a novel physiological substrate of SIRT2. SIRT2 interacted with PARP1 at the PARP-A-helical domain and deacetylated the K249 residue of PARP1. Furthermore, SIRT2 promoted ubiquitination of the K249 residue of PARP1 via mobilization of the E3 ubiquitin ligase WW domain-containing protein 2 (WWP2), which led to proteasome-mediated degradation of PARP1. Knockout of SIRT2 in mice and cells increased PARP1 acetylation and decreased PARP1 ubiquitination, which in turn aggravated oxidative stress-induced vascular injury and remodeling. Conversely, overexpression of SIRT2 in mice and cells decreased PARP1 acetylation, increased PARP1 ubiquitination, and relieved oxidative stress-induced vascular injury and remodeling. Overall, this study revealed a previously unrecognized mechanistic link between SIRT2 and PARP1 in the regulation of oxidative stress-induced vascular injury.
中文翻译:

SIRT2对PARP1的去乙酰化依赖性调节决定了PARP1在氧化应激诱导的血管损伤中的泛素化
聚(ADP-核糖)聚合酶 1 (PARP1) 在心血管疾病中具有重要的调节作用。然而,抑制PARP1活性并不能显着改善心血管疾病的临床结局,这表明PARP1在心血管疾病中的调控机制尚不清楚。在这里,我们专注于 PARP1 的去乙酰化调控机制和 PARP1 翻译后修饰的串扰。我们发现了去乙酰化酶 Sirtuin 2 (SIRT2) 和 PARP1 在血管损伤中的关键分子相互作用和蛋白质修饰。结果表明,SIRT2参与了这一过程,氧化应激损伤因子PARP1是SIRT2的新型生理底物。SIRT2 在 PARP-A 螺旋结构域与 PARP1 相互作用并使 PARP1 的 K249 残基去乙酰化。此外,SIRT2 通过动员 E3 泛素连接酶 WW 结构域的蛋白质 2 (WWP2) 促进 PARP1 的 K249 残基泛素化,从而导致蛋白酶体介导的 PARP1 降解。在小鼠和细胞中敲除 SIRT2 会增加 PARP1 乙酰化并降低 PARP1 泛素化,这反过来又会加重氧化应激诱导的血管损伤和重塑。相反,SIRT2 在小鼠和细胞中的过表达降低了 PARP1 乙酰化,增加了 PARP1 泛素化,并缓解了氧化应激诱导的血管损伤和重塑。总体而言,这项研究揭示了 SIRT2 和 PARP1 之间在调节氧化应激诱导的血管损伤方面以前未被认识的机制联系。这导致蛋白酶体介导的 PARP1 降解。在小鼠和细胞中敲除 SIRT2 会增加 PARP1 乙酰化并降低 PARP1 泛素化,这反过来又会加重氧化应激诱导的血管损伤和重塑。相反,SIRT2 在小鼠和细胞中的过表达降低了 PARP1 乙酰化,增加了 PARP1 泛素化,并缓解了氧化应激诱导的血管损伤和重塑。总体而言,这项研究揭示了 SIRT2 和 PARP1 之间在调节氧化应激诱导的血管损伤方面以前未被认识的机制联系。这导致蛋白酶体介导的 PARP1 降解。在小鼠和细胞中敲除 SIRT2 会增加 PARP1 乙酰化并降低 PARP1 泛素化,这反过来又会加重氧化应激诱导的血管损伤和重塑。相反,SIRT2 在小鼠和细胞中的过表达降低了 PARP1 乙酰化,增加了 PARP1 泛素化,并缓解了氧化应激诱导的血管损伤和重塑。总体而言,这项研究揭示了 SIRT2 和 PARP1 之间在调节氧化应激诱导的血管损伤方面以前未被认识的机制联系。增加 PARP1 泛素化,并缓解氧化应激诱导的血管损伤和重塑。总体而言,这项研究揭示了 SIRT2 和 PARP1 之间在调节氧化应激诱导的血管损伤方面以前未被认识的机制联系。增加 PARP1 泛素化,并缓解氧化应激诱导的血管损伤和重塑。总体而言,这项研究揭示了 SIRT2 和 PARP1 之间在调节氧化应激诱导的血管损伤方面以前未被认识的机制联系。











































 京公网安备 11010802027423号
京公网安备 11010802027423号