Journal of Colloid and Interface Science ( IF 9.4 ) Pub Date : 2021-09-20 , DOI: 10.1016/j.jcis.2021.09.081 Yang-Wen Wu 1 , Xin-Yue Zhou 1 , Qi Cai 1 , Zhuang Hu 1 , Teng-Ge Mi 1 , Bing Zhang 1 , Li Zhao 1 , Qiang Lu 1
|
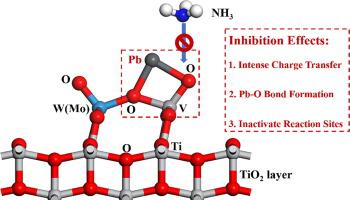
Lead (Pb) species trigger serious poisoning of selective catalytic reduction (SCR) catalysts. To improve the Pb resistance ability, revealing the impact mechanism of Pb species on the commercial SCR catalysts from a molecular level is of great significance. Herein, first-principles calculations were applied to unveil the Pb adsorption mechanism on the vanadium-based catalysts, the results were also compared with the previous experimental findings. The intrinsic interaction mechanism between Pb and catalyst components was interpreted by clarifying the change of the catalyst electronic structures (including charge transfer, bond formation situations, and active sites reactivities). It is found that the adsorption of Pb species belongs to chemisorption, evident electron transfer with the catalyst surface is inspected and intense charge transfer indicates strong adsorption. A remarkable interaction with the V = O active sites occurs and stable Pb-O bonds are formed, which significantly changes the electronic structures of the V = O sites and inhibits the NH3 adsorption, thus suppressing the SCR activity. Finally, thermodynamic analysis was applied to elucidate the temperature influence on Pb adsorption. It is found that Pb adsorption on catalysts cannot proceed spontaneously over 500 K. At higher temperatures the adsorption is inhibited and the Pb species become less stable, which partially explains why the Pb-poisoning effect at high temperatures is relatively moderate than that at low temperatures.
中文翻译:

基于第一性原理研究的铅物种与钒基催化剂相互作用的内在机制洞察
铅 (Pb) 物种会引发选择性催化还原 (SCR) 催化剂的严重中毒。为了提高抗铅能力,从分子水平揭示铅物种对商用SCR催化剂的影响机制具有重要意义。在此,应用第一性原理计算揭示了钒基催化剂上 Pb 的吸附机理,并将结果与之前的实验结果进行了比较。通过阐明催化剂电子结构的变化(包括电荷转移、键形成情况和活性位点反应性)来解释 Pb 和催化剂组分之间的内在相互作用机制。发现Pb物种的吸附属于化学吸附,检查与催化剂表面明显的电子转移,强烈的电荷转移表明强吸附。与 V = O 活性位点发生显着的相互作用并形成稳定的 Pb-O 键,这显着改变了 V = O 位点的电子结构并抑制了 NH3吸附,从而抑制SCR活性。最后,应用热力学分析阐明温度对 Pb 吸附的影响。发现超过 500 K 时,催化剂上的 Pb 吸附不能自发进行。在较高温度下,吸附受到抑制,Pb 物质变得不稳定,这部分解释了为什么高温下的铅中毒效应比低温下的相对温和.











































 京公网安备 11010802027423号
京公网安备 11010802027423号