Journal of Allergy and Clinical Immunology ( IF 11.4 ) Pub Date : 2021-09-20 , DOI: 10.1016/j.jaci.2021.08.027 Mingzhu Jin 1 , Simon Watkins 2 , Yolanda Larriba 3 , Callen Wallace 2 , Claudette St Croix 2 , Xiuxia Zhou 4 , Jinming Zhao 4 , Shyamal Peddada 5 , Sally E Wenzel 6
|
Background
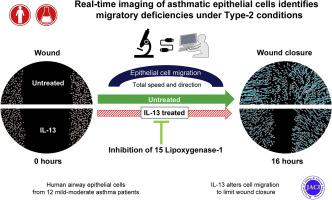
The epithelium is increasingly recognized as a pathologic contributor to asthma and its phenotypes. Although delayed wound closure by asthmatic epithelial cells is consistently observed, underlying mechanisms remain poorly understood, partly due to difficulties in studying dynamic physiologic processes involving polarized multilayered cell systems. Although type-2 immunity has been suggested to play a role, the mechanisms by which repair is diminished are unclear.
Objectives
This study sought to develop and utilize primary multilayered polarized epithelial cell systems, derived from patients with asthma, to evaluate cell migration in response to wounding under type-2 and untreated conditions.
Methods
A novel wounding device for multilayered polarized cells, along with time-lapse live cell/real-time confocal imaging were evaluated under IL-13 and untreated conditions. The influence of inhibition of 15 lipoxygenase (15LO1), a type-2 enzyme, on the process was also addressed. Cell migration patterns were analyzed by high-dimensional frequency modulated Möbius for statistical comparisons.
Results
IL-13 stimulation negatively impacts wound healing by altering the total speed, directionality, and acceleration of individual cells. Inhibition 15LO1 partially improved the wound repair through improving total speed.
Conclusions
Migration abnormalities contributed to markedly slower wound closure of IL-13 treated cells, which was modestly reversed by 15LO1 inhibition, suggesting its potential as an asthma therapeutic target. These novel methodologies offer new ways to dynamically study cell movements and identify contributing pathologic processes.
中文翻译:

哮喘上皮细胞的实时成像可识别 2 型条件下的迁移缺陷
背景
上皮越来越被认为是哮喘及其表型的病理贡献者。尽管一直观察到哮喘上皮细胞导致伤口闭合延迟,但其潜在机制仍然知之甚少,部分原因是研究涉及极化多层细胞系统的动态生理过程存在困难。尽管有人建议 2 型免疫发挥作用,但修复减少的机制尚不清楚。
目标
本研究旨在开发和利用源自哮喘患者的原发性多层极化上皮细胞系统,以评估细胞迁移在 2 型和未治疗条件下对创伤的反应。
方法
在 IL-13 和未处理条件下评估了一种用于多层极化细胞的新型创伤装置,以及延时活细胞/实时共聚焦成像。还讨论了抑制 15 脂氧合酶 (15LO1)(一种 2 型酶)对该过程的影响。通过高维频率调制莫比乌斯分析细胞迁移模式以进行统计比较。
结果
IL-13 刺激通过改变单个细胞的总速度、方向性和加速度对伤口愈合产生负面影响。抑制 15LO1 通过提高总速度部分改善了伤口修复。
结论
迁移异常导致经 IL-13 处理的细胞的伤口闭合明显变慢,而 15LO1 抑制可适度逆转,表明其作为哮喘治疗靶点的潜力。这些新颖的方法提供了动态研究细胞运动和识别病理过程的新方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号