International Journal of Hydrogen Energy ( IF 7.2 ) Pub Date : 2021-09-20 , DOI: 10.1016/j.ijhydene.2021.09.003 A. Rajapriya 1 , S. Keerthana 1 , A. Rebekah 1 , C. Viswanathan 1 , N. Ponpandian 1
|
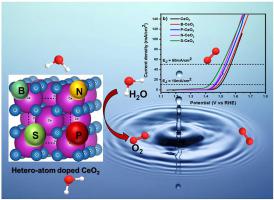
Increasing worldwide energy consumption has prompted considerable study into energy generation and energy storage systems in recent years. Chemical fuels may be produced efficiently via electrocatalytic water splitting, which uses electric and solar power. The development of efficient anodic electrocatalysts for efficient oxygen evolution reaction (OER) is a greater concern of present energy research. Cerium oxide (CeO2) are promising electrocatalysts that exhibit outstanding OER but their reduced stability obstructs the practical application. A novel strategy was established to construct an effective catalyst of heteroatom (N, B, P and S) doped CeO2 matrix were prepared. Moreover, the doping of heteroatoms into the CeO2 matrix processes the improved electronic conductivity, reactive sites, increases the electrochemical catalytic activity, which enhances the water oxidation reaction. Consequently, well-suited alkaline electrolysers were brought together for water oxidation to ideal OER electrocatalytic activity. The OER activity of the electrocatalysts follows the order of S–CeO2 (190 mV@10 mA cm−2), N– CeO2 (220 mV @10 mA cm−2), P– CeO2 (230 mV @10 mA cm−2), B–CeO2 (250 mV @10 mA cm−2) and CeO2 (260 mV @10 mA cm−2) in 1 M of KOH. From the kinetics analysis, Tafel slope value achieved for catalysts CeO2, B–CeO2, P–CeO2, N–CeO2 and S–CeO2 are 142 mV dec−1,121 mV dec−1, 102 mV dec−1, 98 mV dec−1 and 83 mV dec−1 respectively. These results validate that the S–CeO2 electrode is prominent for OER performance with the requirement of cell voltage of 1.42 V at 10 mA cm−2 current density. In addition, sulphur doped CeO2 relatively have excellent stability through chrono-potentiometric analysis lasting for 20 h. Although the heteroatoms doped CeO2 is acts as anode material, the preparation method is widespread, which will reduce the synthesis cost and streamline the preparation of electrode for OER. This research effort delivers a complete advantage for the development of robust, environmentally friendly and highly dynamic electrocatalysts for OER activity.
中文翻译:

富氧空位促进杂原子(B、P、N 和 S)掺杂的 CeO2:碱性介质中析氧反应 (OER) 的具有挑战性的电催化剂
近年来,全球能源消耗的增加促使人们对能源生产和储能系统进行了大量研究。化学燃料可以通过使用电力和太阳能的电催化水分解有效地生产。用于高效析氧反应(OER)的高效阳极电催化剂的开发是当前能源研究的一个更大的关注点。氧化铈(CeO 2)是一种很有前途的电催化剂,具有出色的OER,但其稳定性降低阻碍了实际应用。建立了一种构建杂原子(N、B、P 和S)掺杂CeO 2基体的有效催化剂的新策略。此外,杂原子掺杂到 CeO 2基体处理改进的电子导电性、反应位点,增加电化学催化活性,从而增强水氧化反应。因此,将非常适合的碱性电解槽组合在一起,将水氧化至理想的 OER 电催化活性。电催化剂的 OER 活性遵循 S–CeO 2 (190 mV@10 mA cm -2 )、N– CeO 2 (220 mV @10 mA cm -2 )、P– CeO 2 (230 mV @10 mA) cm -2 )、B–CeO 2 (250 mV @10 mA cm -2 ) 和 CeO 2 (260 mV @10 mA cm -2) 在 1 M 的 KOH 中。根据动力学分析,催化剂 CeO 2、B-CeO 2、 P-CeO 2、N-CeO 2和 S-CeO 2 的塔菲尔斜率值为142 mV dec -1、121 mV dec -1、102 mV dec - 1,98毫伏癸-1和83毫伏癸-1分别。这些结果验证了 S-CeO 2电极对 OER 性能的显着影响,在 10 mA cm -2电流密度下需要 1.42 V 的电池电压。此外,硫掺杂的 CeO 2通过持续20小时的计时电位分析,相对具有出色的稳定性。虽然杂原子掺杂的CeO 2作为负极材料,但制备方法广泛,这将降低合成成本并简化OER电极的制备。这项研究工作为开发用于 OER 活性的坚固、环保和高动态电催化剂提供了完整的优势。



























 京公网安备 11010802027423号
京公网安备 11010802027423号