当前位置:
X-MOL 学术
›
Energy Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Study of CexFe1−xO2 as a Reducible Oxide for the Thermal Hydrogen Production from Water
Energy Technology ( IF 3.6 ) Pub Date : 2021-09-18 , DOI: 10.1002/ente.202100491 S. Al-Taweel 1 , M.A. Nadeem 1 , Hicham Idriss 1, 2
Energy Technology ( IF 3.6 ) Pub Date : 2021-09-18 , DOI: 10.1002/ente.202100491 S. Al-Taweel 1 , M.A. Nadeem 1 , Hicham Idriss 1, 2
Affiliation
|
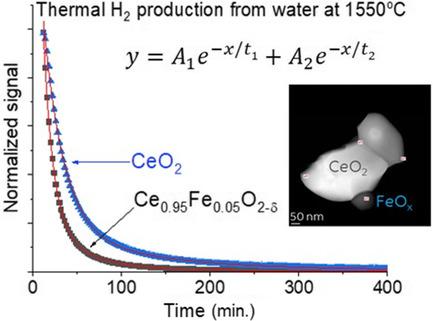
Thermal water splitting over 1550 °C-reduced CeO2 and Ce0.95Fe0.05O2−δ is studied. Hydrogen production over Ce0.95Fe0.05O2−δ was found to be equal to which is higher than that observed on CeO2. The reaction kinetics for Ce0.95Fe0.05O2−δ is also found to be faster. The oxides are studied by X-ray diffraction, temperature programmed reduction, and scanning transmission electron microscopy (STEM). XRD results show that Fe is substituted for Ce4+ in the as prepared oxides. Fe3+ cations substitution also decreases the CeO2 crystallites size. Heating to 1100 °C increases their size, although the Fe-containing oxide still shows smaller crystallites when compared with CeO2 alone. The activation energy for surface reduction of CeO2, extracted from TPR, is found to be slightly higher (1.58 eV) than that of bulk reduction (1.43 eV). While high resolution TEM and electron energy loss spectroscopy before reaction show that Fe cations are homogenously distributed, those after reaction show in addition to the growth of the crystallites size, Fe segregation to the edges of the crystals, although no detachment of Fe oxide particles is seen. The mechanism of water dissociative adsorption and hydrogen re-combinative desorption is discussed in which the role of Ce–O–Fe sites is considered.
中文翻译:

CexFe1−xO2 作为从水中热制氢的可还原氧化物的研究
研究了通过 1550 °C 还原的 CeO 2和 Ce 0.95 Fe 0.05 O 2- δ 的热水分解。发现Ce 0.95 Fe 0.05 O 2− δ 上的氢气产量等于这高于在 CeO 2上观察到的。还发现Ce 0.95 Fe 0.05 O 2- δ的反应动力学更快。通过 X 射线衍射、程序升温还原和扫描透射电子显微镜 (STEM) 研究氧化物。XRD结果表明在制备的氧化物中Fe取代了Ce 4+。Fe 3+阳离子取代也降低了CeO 2微晶尺寸。加热到 1100 °C 会增加它们的尺寸,尽管与单独的CeO 2相比,含 Fe 氧化物仍然显示出更小的微晶。CeO 2表面还原的活化能从 TPR 中提取的 被发现略高于体还原(1.43 eV)(1.58 eV)。虽然反应前的高分辨率 TEM 和电子能量损失光谱表明 Fe 阳离子分布均匀,但反应后的那些除了微晶尺寸的增长外,Fe 偏析到晶体的边缘,虽然没有 Fe 氧化物颗粒的分离看到了。讨论了水解离吸附和氢复合解吸的机理,其中考虑了 Ce-O-Fe 位点的作用。
更新日期:2021-09-18
中文翻译:

CexFe1−xO2 作为从水中热制氢的可还原氧化物的研究
研究了通过 1550 °C 还原的 CeO 2和 Ce 0.95 Fe 0.05 O 2- δ 的热水分解。发现Ce 0.95 Fe 0.05 O 2− δ 上的氢气产量等于这高于在 CeO 2上观察到的。还发现Ce 0.95 Fe 0.05 O 2- δ的反应动力学更快。通过 X 射线衍射、程序升温还原和扫描透射电子显微镜 (STEM) 研究氧化物。XRD结果表明在制备的氧化物中Fe取代了Ce 4+。Fe 3+阳离子取代也降低了CeO 2微晶尺寸。加热到 1100 °C 会增加它们的尺寸,尽管与单独的CeO 2相比,含 Fe 氧化物仍然显示出更小的微晶。CeO 2表面还原的活化能从 TPR 中提取的 被发现略高于体还原(1.43 eV)(1.58 eV)。虽然反应前的高分辨率 TEM 和电子能量损失光谱表明 Fe 阳离子分布均匀,但反应后的那些除了微晶尺寸的增长外,Fe 偏析到晶体的边缘,虽然没有 Fe 氧化物颗粒的分离看到了。讨论了水解离吸附和氢复合解吸的机理,其中考虑了 Ce-O-Fe 位点的作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号