Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
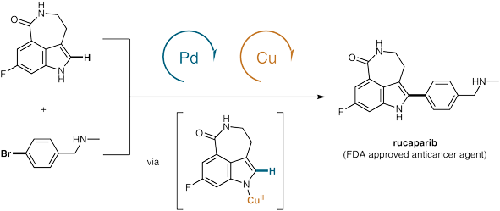
Late-Stage C–H Arylation of Azepinoindole via Pd/Cu Catalysis: A Step Efficient and Convergent Synthesis of Rucaparib
Synthesis ( IF 2.2 ) Pub Date : 2021-09-17 , DOI: 10.1055/s-0037-1610784 Dirk De Vos , Igor Beckers , Galahad O’Rourke
中文翻译:

通过 Pd/Cu 催化氮杂吲哚的后期 C-H 芳基化:Rucaparib 的一步高效和收敛合成
更新日期:2021-09-19
Synthesis ( IF 2.2 ) Pub Date : 2021-09-17 , DOI: 10.1055/s-0037-1610784 Dirk De Vos , Igor Beckers , Galahad O’Rourke
|
The C–H arylation of indoles holds the promise to shorten synthetic routes in the production of pharmaceuticals. However, late-stage C–H activation reactions often rely on the presence of protecting groups or stoichiometric metal additives. The regiospecific C–H arylation of a highly functionalized azepino[5,3,4-cd]indole scaffold lacking directing groups via Pd(II) and Cu(II) co-catalysis is reported. The direct C–H coupling was demonstrated in the convergent synthesis of rucaparib, an FDA approved anticancer drug.
中文翻译:

通过 Pd/Cu 催化氮杂吲哚的后期 C-H 芳基化:Rucaparib 的一步高效和收敛合成
吲哚的 C-H 芳基化有望缩短药物生产中的合成路线。然而,后期 C-H 活化反应通常依赖于保护基团或化学计量金属添加剂的存在。据报道,高度官能化的氮杂[5,3,4- cd ]吲哚支架通过Pd(II)和Cu(II)共催化进行区域特异性C-H芳基化,该支架缺乏导向基团。直接 C-H 偶联在 FDA 批准的抗癌药物 rucaparib 的收敛合成中得到证明。











































 京公网安备 11010802027423号
京公网安备 11010802027423号