当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Tetraarylpyrrole-Based Phosphine Ligand for the Palladium-Catalyzed Amination of Aryl Chlorides
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2021-09-17 , DOI: 10.1002/adsc.202100731 Masahiro Sai 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2021-09-17 , DOI: 10.1002/adsc.202100731 Masahiro Sai 1
Affiliation
|
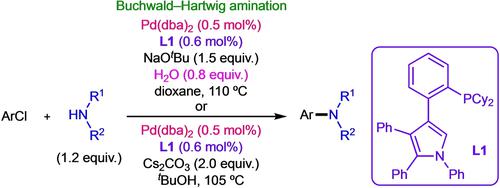
A tetraarylpyrrole-based phosphine ligand L1 in combination with Pd(dba)2 provided a catalyst for the Buchwald-Hartwig amination reaction. A variety of amines were rapidly coupled with aryl chlorides at a Pd loading of 0.5 mol%. The selective monoarylation of aliphatic primary amines was achieved in the presence of 0.8 equiv. water. Comparison experiments were also conducted, which revealed that the catalytic activity of L1 is superior to representative phosphine ligands in the Pd-catalyzed C−N coupling of various amines.
中文翻译:

用于钯催化胺化芳基氯化物的四芳基吡咯基膦配体
基于四芳基吡咯的膦配体L1与 Pd(dba) 2结合为 Buchwald-Hartwig 胺化反应提供了催化剂。多种胺以 0.5 mol% 的 Pd 负载量与芳基氯快速偶联。在 0.8 当量的存在下实现了脂肪族伯胺的选择性单芳基化。水。还进行了比较实验,结果表明L1的催化活性优于 Pd 催化的各种胺的 CN 偶联中的代表性膦配体。
更新日期:2021-09-17
中文翻译:

用于钯催化胺化芳基氯化物的四芳基吡咯基膦配体
基于四芳基吡咯的膦配体L1与 Pd(dba) 2结合为 Buchwald-Hartwig 胺化反应提供了催化剂。多种胺以 0.5 mol% 的 Pd 负载量与芳基氯快速偶联。在 0.8 当量的存在下实现了脂肪族伯胺的选择性单芳基化。水。还进行了比较实验,结果表明L1的催化活性优于 Pd 催化的各种胺的 CN 偶联中的代表性膦配体。











































 京公网安备 11010802027423号
京公网安备 11010802027423号