当前位置:
X-MOL 学术
›
Adv. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Sono-Controllable and ROS-Sensitive CRISPR-Cas9 Genome Editing for Augmented/Synergistic Ultrasound Tumor Nanotherapy
Advanced Materials ( IF 27.4 ) Pub Date : 2021-09-18 , DOI: 10.1002/adma.202104641 Yinying Pu 1 , Haohao Yin 1 , Caihong Dong 2 , Huijing Xiang 3 , Wencheng Wu 4 , Bangguo Zhou 1 , Dou Du 1 , Yu Chen 3 , Huixiong Xu 1
Advanced Materials ( IF 27.4 ) Pub Date : 2021-09-18 , DOI: 10.1002/adma.202104641 Yinying Pu 1 , Haohao Yin 1 , Caihong Dong 2 , Huijing Xiang 3 , Wencheng Wu 4 , Bangguo Zhou 1 , Dou Du 1 , Yu Chen 3 , Huixiong Xu 1
Affiliation
|
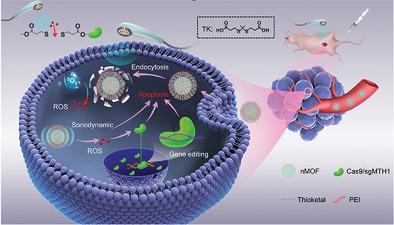
The potential of the cluster regularly interspaced short palindromic repeat (CRISPR)-associated protein 9 (Cas9)-based therapeutic genome editing is severely hampered by the difficulties in precise regulation of the in vivo activity of the CRISPR-Cas9 system. Herein, sono-controllable and reactive oxygen species (ROS)-sensitive sonosensitizer-integrated metal–organic frameworks (MOFs), denoted as P/M@CasMTH1, are developed for augmented sonodynamic therapy (SDT) efficacy using the genome-editing technology. P/M@CasMTH1 nanoparticles comprise singlet oxygen (1O2)-generating MOF structures anchored with CRISPR-Cas9 systems via 1O2-cleavable linkers, which serve not only as a delivery vector of CRISPR-Cas9 targeting MTH1, but also as a sonoregulator to spatiotemporally activate the genome editing. P/M@CasMTH1 escapes from the lysosomes, harvests the ultrasound (US) energy and converts it into abundant 1O2 to induce SDT. The generated ROS subsequently trigger cleavage of ROS-responsive thioether bonds, thus inducing controllable release of the CRISPR-Cas9 system and initiation of genome editing. The genomic disruption of MTH1 conspicuously augments the therapeutic efficacy of SDT by destroying the self-defense system in tumor cells, thereby causing cellular apoptosis and tumor suppression. This therapeutic strategy for synergistic MTH1 disruption and abundant 1O2 generation provides a paradigm for augmenting SDT efficacy based on the emerging nanomedicine-enabled genome-editing technology.
中文翻译:

用于增强/协同超声肿瘤纳米治疗的声波可控且 ROS 敏感的 CRISPR-Cas9 基因组编辑
基于簇规则间隔短回文重复序列 (CRISPR) 相关蛋白 9 (Cas9) 的治疗性基因组编辑的潜力因 CRISPR-Cas9 系统体内活性的精确调节困难而受到严重阻碍。在此,开发了声可控和活性氧(ROS)敏感的声敏剂集成金属有机框架(MOF),表示为P/M@CasMTH1,利用基因组编辑技术增强声动力疗法(SDT)功效。 P/M@CasMTH1 纳米颗粒包含产生单线态氧 ( 1 O 2 ) 的 MOF 结构,通过1 O 2可裂解接头锚定于 CRISPR-Cas9 系统,其不仅可作为靶向 MTH1 的 CRISPR-Cas9 的递送载体,而且还可作为声波调节器,用于时空激活基因组编辑。 P/M@CasMTH1 从溶酶体中逃逸,收集超声波 (US) 能量并将其转化为丰富的1 O 2以诱导 SDT。生成的 ROS 随后触发 ROS 响应性硫醚键的裂解,从而诱导 CRISPR-Cas9 系统的可控释放并启动基因组编辑。 MTH1基因组的破坏通过破坏肿瘤细胞的自我防御系统,从而引起细胞凋亡和肿瘤抑制,显着增强了SDT的治疗效果。这种协同 MTH1 破坏和丰富的1 O 2生成的治疗策略为基于新兴的纳米医学基因组编辑技术增强 SDT 功效提供了范例。
更新日期:2021-11-09
中文翻译:

用于增强/协同超声肿瘤纳米治疗的声波可控且 ROS 敏感的 CRISPR-Cas9 基因组编辑
基于簇规则间隔短回文重复序列 (CRISPR) 相关蛋白 9 (Cas9) 的治疗性基因组编辑的潜力因 CRISPR-Cas9 系统体内活性的精确调节困难而受到严重阻碍。在此,开发了声可控和活性氧(ROS)敏感的声敏剂集成金属有机框架(MOF),表示为P/M@CasMTH1,利用基因组编辑技术增强声动力疗法(SDT)功效。 P/M@CasMTH1 纳米颗粒包含产生单线态氧 ( 1 O 2 ) 的 MOF 结构,通过1 O 2可裂解接头锚定于 CRISPR-Cas9 系统,其不仅可作为靶向 MTH1 的 CRISPR-Cas9 的递送载体,而且还可作为声波调节器,用于时空激活基因组编辑。 P/M@CasMTH1 从溶酶体中逃逸,收集超声波 (US) 能量并将其转化为丰富的1 O 2以诱导 SDT。生成的 ROS 随后触发 ROS 响应性硫醚键的裂解,从而诱导 CRISPR-Cas9 系统的可控释放并启动基因组编辑。 MTH1基因组的破坏通过破坏肿瘤细胞的自我防御系统,从而引起细胞凋亡和肿瘤抑制,显着增强了SDT的治疗效果。这种协同 MTH1 破坏和丰富的1 O 2生成的治疗策略为基于新兴的纳米医学基因组编辑技术增强 SDT 功效提供了范例。











































 京公网安备 11010802027423号
京公网安备 11010802027423号